Adenosine Receptor mRNA Expression in Frontal Cortical Neurons in Schizophrenia
- PMID: 38201235
- PMCID: PMC10778287
- DOI: 10.3390/cells13010032
Adenosine Receptor mRNA Expression in Frontal Cortical Neurons in Schizophrenia
Abstract
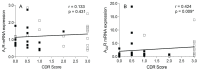
Schizophrenia is a devastating neuropsychiatric disorder associated with the dysregulation of glutamate and dopamine neurotransmitter systems. The adenosine system is an important neuroregulatory system in the brain that modulates glutamate and dopamine signaling via the ubiquitously expressed adenosine receptors; however, adenosine A1 and A2A receptor (A1R and A2AR) mRNA expression is poorly understood in specific cell subtypes in the frontal cortical brain regions implicated in this disorder. In this study, we assayed A1R and A2AR mRNA expression via qPCR in enriched populations of pyramidal neurons, which were isolated from postmortem anterior cingulate cortex (ACC) tissue from schizophrenia (n = 20) and control (n = 20) subjects using laser microdissection (LMD). A1R expression was significantly increased in female schizophrenia subjects compared to female control subjects (t(13) = -4.008, p = 0.001). A1R expression was also significantly decreased in female control subjects compared to male control subjects, suggesting sex differences in basal A1R expression (t(17) = 2.137, p = 0.047). A significant, positive association was found between dementia severity (clinical dementia rating (CDR) scores) and A2AR mRNA expression (Spearman's r = 0.424, p = 0.009). A2AR mRNA expression was significantly increased in unmedicated schizophrenia subjects, suggesting that A2AR expression may be normalized by chronic antipsychotic treatment (F(1,14) = 9.259, p = 0.009). Together, these results provide novel insights into the neuronal expression of adenosine receptors in the ACC in schizophrenia and suggest that receptor expression changes may be sex-dependent and associated with cognitive decline in these subjects.
Keywords: adenosine receptors; anterior cingulate cortex; neuromodulation; pyramidal neurons; schizophrenia; transcript expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

