The Role of Apoptosis and Oxidative Stress in a Cell Spheroid Model of Calcific Aortic Valve Disease
- PMID: 38201249
- PMCID: PMC10778193
- DOI: 10.3390/cells13010045
The Role of Apoptosis and Oxidative Stress in a Cell Spheroid Model of Calcific Aortic Valve Disease
Abstract
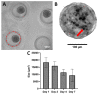
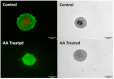
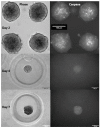
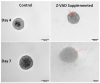
Calcific aortic valve disease (CAVD) is the most common heart valve disease among aging populations. There are two reported pathways of CAVD: osteogenic and dystrophic, the latter being more prevalent. Current two-dimensional (2D) in vitro CAVD models have shed light on the disease but lack three-dimensional (3D) cell-ECM interactions, and current 3D models require osteogenic media to induce calcification. The goal of this work is to develop a 3D dystrophic calcification model. We hypothesize that, as with 2D cell-based CAVD models, programmed cell death (apoptosis) is integral to calcification. We model the cell aggregation observed in CAVD by creating porcine valvular interstitial cell spheroids in agarose microwells. Upon culture in complete growth media (DMEM with serum), calcium nodules form in the spheroids within a few days. Inhibiting apoptosis with Z-VAD significantly reduced calcification, indicating that the calcification observed in this model is dystrophic rather than osteogenic. To determine the relative roles of oxidative stress and extracellular matrix (ECM) production in the induction of apoptosis and subsequent calcification, the media was supplemented with antioxidants with differing effects on ECM formation (ascorbic acid (AA), Trolox, or Methionine). All three antioxidants significantly reduced calcification as measured by Von Kossa staining, with the percentages of calcification per area of AA, Trolox, Methionine, and the non-antioxidant-treated control on day 7 equaling 0.17%, 2.5%, 6.0%, and 7.7%, respectively. As ZVAD and AA almost entirely inhibit calcification, apoptosis does not appear to be caused by a lack of diffusion of oxygen and metabolites within the small spheroids. Further, the observation that AA treatment reduces calcification significantly more than the other antioxidants indicates that the ECM stimulatory effect of AA plays a role inhibiting apoptosis and calcification in the spheroids. We conclude that, in this 3D in vitro model, both oxidative stress and ECM production play crucial roles in dystrophic calcification and may be viable therapeutic targets for preventing CAVD.
Keywords: antioxidant; apoptosis; calcific aortic heart valve disease; oxidative stress; valvular interstitial cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Transforming growth factor-β1 promotes fibrosis but attenuates calcification of valvular tissue applied as a three-dimensional calcific aortic valve disease model.Am J Physiol Heart Circ Physiol. 2020 Nov 1;319(5):H1123-H1141. doi: 10.1152/ajpheart.00651.2019. Epub 2020 Sep 28. Am J Physiol Heart Circ Physiol. 2020. PMID: 32986963
-
Simulation of early calcific aortic valve disease in a 3D platform: A role for myofibroblast differentiation.J Mol Cell Cardiol. 2016 May;94:13-20. doi: 10.1016/j.yjmcc.2016.03.004. Epub 2016 Mar 17. J Mol Cell Cardiol. 2016. PMID: 26996755 Free PMC article.
-
COX-2 Is Downregulated in Human Stenotic Aortic Valves and Its Inhibition Promotes Dystrophic Calcification.Int J Mol Sci. 2020 Nov 24;21(23):8917. doi: 10.3390/ijms21238917. Int J Mol Sci. 2020. PMID: 33255450 Free PMC article.
-
Contribution of Oxidative Stress (OS) in Calcific Aortic Valve Disease (CAVD): From Pathophysiology to Therapeutic Targets.Cells. 2022 Aug 27;11(17):2663. doi: 10.3390/cells11172663. Cells. 2022. PMID: 36078071 Free PMC article. Review.
-
Extracellular Matrix in Calcific Aortic Valve Disease: Architecture, Dynamic and Perspectives.Int J Mol Sci. 2021 Jan 18;22(2):913. doi: 10.3390/ijms22020913. Int J Mol Sci. 2021. PMID: 33477599 Free PMC article. Review.
Cited by
-
Towards a More Objective and High-throughput Spheroid Invasion Assay Quantification Method.bioRxiv [Preprint]. 2024 Oct 18:2024.06.27.600893. doi: 10.1101/2024.06.27.600893. bioRxiv. 2024. Update in: Sci Rep. 2024 Dec 28;14(1):31007. doi: 10.1038/s41598-024-82191-3. PMID: 39005385 Free PMC article. Updated. Preprint.
-
Unveiling the Angiogenic Potential and Functional Decline of Valve Interstitial Cells During Calcific Aortic Valve Stenosis Progression.J Cell Mol Med. 2025 Apr;29(7):e70511. doi: 10.1111/jcmm.70511. J Cell Mol Med. 2025. PMID: 40159645 Free PMC article.
-
Revealing the mechanisms of warfarin-induced vascular calcification through metabolomics and network toxicology.Front Pharmacol. 2025 Jun 9;16:1554987. doi: 10.3389/fphar.2025.1554987. eCollection 2025. Front Pharmacol. 2025. PMID: 40552162 Free PMC article.
-
Towards a more objective and high-throughput spheroid invasion assay quantification method.Sci Rep. 2024 Dec 28;14(1):31007. doi: 10.1038/s41598-024-82191-3. Sci Rep. 2024. PMID: 39730859 Free PMC article.
References
-
- Yutzey K.E., Demer L.L., Body S.C., Huggins G.S., Towler D.A., Giachelli C.M., Hofmann-Bowman M.A., Mortlock D.P., Rogers M.B., Sadeghi M.M., et al. Calcific aortic valve disease: A consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler. Thromb. Vasc. Biol. 2014;34:2387–2393. doi: 10.1161/ATVBAHA.114.302523. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

