Targeted Inhibitors of EGFR: Structure, Biology, Biomarkers, and Clinical Applications
- PMID: 38201251
- PMCID: PMC10778338
- DOI: 10.3390/cells13010047
Targeted Inhibitors of EGFR: Structure, Biology, Biomarkers, and Clinical Applications
Abstract
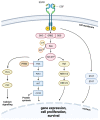
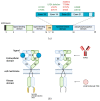
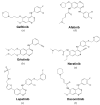
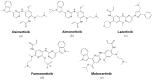
Members of the EGFR family of tyrosine kinase receptors are major regulators of cellular proliferation, differentiation, and survival. In humans, abnormal activation of EGFR is associated with the development and progression of many cancer types, which makes it an attractive target for molecular-guided therapy. Two classes of EGFR-targeted cancer therapeutics include monoclonal antibodies (mAbs), which bind to the extracellular domain of EGFR, and tyrosine kinase inhibitors (TKIs), which mostly target the intracellular part of EGFR and inhibit its activity in molecular signaling. While EGFR-specific mAbs and three generations of TKIs have demonstrated clinical efficacy in various settings, molecular evolution of tumors leads to apparent and sometimes inevitable resistance to current therapeutics, which highlights the need for deeper research in this field. Here, we tried to provide a comprehensive and systematic overview of the rationale, molecular mechanisms, and clinical significance of the current EGFR-targeting drugs, highlighting potential candidate molecules in development. We summarized the underlying mechanisms of resistance and available personalized predictive approaches that may lead to improved efficacy of EGFR-targeted therapies. We also discuss recent developments and the use of specific therapeutic strategies, such as multi-targeting agents and combination therapies, for overcoming cancer resistance to EGFR-specific drugs.
Keywords: EGFR mutations; EGFR-targeting drugs; HER-targeted drugs; epidermal growth factor receptor (EGFR); secondary resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Critical update and emerging trends in epidermal growth factor receptor targeting in cancer.J Clin Oncol. 2005 Apr 10;23(11):2445-59. doi: 10.1200/JCO.2005.11.890. Epub 2005 Mar 7. J Clin Oncol. 2005. PMID: 15753456 Review.
-
Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers.Pharmacol Res. 2019 Jan;139:395-411. doi: 10.1016/j.phrs.2018.11.014. Epub 2018 Nov 27. Pharmacol Res. 2019. PMID: 30500458 Review.
-
The role of anti-EGFR therapies in EGFR-TKI-resistant advanced non-small cell lung cancer.Cancer Treat Rev. 2024 Jan;122:102664. doi: 10.1016/j.ctrv.2023.102664. Epub 2023 Nov 25. Cancer Treat Rev. 2024. PMID: 38064878 Review.
-
Targeting epidermal growth factor receptor in solid tumors: critical evaluation of the biological importance of therapeutic monoclonal antibodies.Curr Med Chem. 2009;16(29):3797-804. doi: 10.2174/092986709789177984. Curr Med Chem. 2009. PMID: 19747140 Review.
-
Epidermal growth factor receptor inhibition strategies in oncology.Endocr Relat Cancer. 2004 Dec;11(4):689-708. doi: 10.1677/erc.1.00600. Endocr Relat Cancer. 2004. PMID: 15613446 Review.
Cited by
-
Case Report: Grade 4 pneumonitis occurred after thoracic radiotherapy and dacomitinib in a patient with lung adenocarcinoma.Front Oncol. 2025 Feb 25;15:1436134. doi: 10.3389/fonc.2025.1436134. eCollection 2025. Front Oncol. 2025. PMID: 40071087 Free PMC article.
-
Purine-Hydrazone Scaffolds as Potential Dual EGFR/HER2 Inhibitors.Pharmaceuticals (Basel). 2025 Jul 17;18(7):1051. doi: 10.3390/ph18071051. Pharmaceuticals (Basel). 2025. PMID: 40732338 Free PMC article.
-
Lapatinib-induced enhancement of mitochondrial respiration in HER2-positive SK-BR-3 cells: mechanism revealed by analysis of proteomic but not transcriptomic data.Front Mol Biosci. 2024 Sep 30;11:1470496. doi: 10.3389/fmolb.2024.1470496. eCollection 2024. Front Mol Biosci. 2024. PMID: 39403185 Free PMC article.
-
Synthesis, Antitumor Activities, and Apoptosis-Inducing Activities of Schiff's Bases Incorporating Imidazolidine-2,4-dione Scaffold: Molecular Docking Studies and Enzymatic Inhibition Activities.Pharmaceuticals (Basel). 2025 Mar 28;18(4):496. doi: 10.3390/ph18040496. Pharmaceuticals (Basel). 2025. PMID: 40283934 Free PMC article.
-
Translational Insights in the Landscape of Salivary Gland Cancers: Ready for a New Era?Cancers (Basel). 2024 Feb 28;16(5):970. doi: 10.3390/cancers16050970. Cancers (Basel). 2024. PMID: 38473330 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

