CD49f and CD146: A Possible Crosstalk Modulates Adipogenic Differentiation Potential of Mesenchymal Stem Cells
- PMID: 38201259
- PMCID: PMC10778538
- DOI: 10.3390/cells13010055
CD49f and CD146: A Possible Crosstalk Modulates Adipogenic Differentiation Potential of Mesenchymal Stem Cells
Abstract
Background: The lack of appropriate mesenchymal stem cells (MSCs) selection methods has given the challenges for standardized harvesting, processing, and phenotyping procedures of MSCs. Genetic engineering coupled with high-throughput proteomic studies of MSC surface markers arises as a promising strategy to identify stem cell-specific markers. However, the technical limitations are the key factors making it less suitable to provide an appropriate starting material for the screening platform. A more accurate, easily accessible approach is required to solve the issues.
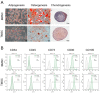
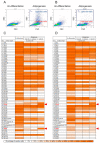
Methods: This study established a high-throughput screening strategy with forward versus side scatter gating to identify the adipogenesis-associated markers of bone marrow-derived MSCs (BMSCs) and tonsil-derived MSCs (TMSCs). We classified the MSC-derived adipogenic differentiated cells into two clusters: lipid-rich cells as side scatter (SSC)-high population and lipid-poor cells as SSC-low population. By screening the expression of 242 cell surface proteins, we identified the surface markers which exclusively found in lipid-rich subpopulation as the specific markers for BMSCs and TMSCs.
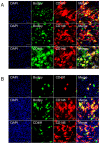
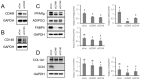
Results: High-throughput screening of the expression of 242 cell surface proteins indicated that CD49f and CD146 were specific for BMSCs and TMSCs. Subsequent immunostaining confirmed the consistent specific expression of CD49f and CD146 and in BMSCs and TMSCs. Enrichment of MSCs by CD49f and CD146 surface markers demonstrated that the simultaneous expression of CD49f and CD146 is required for adipogenesis and osteogenesis of mesenchymal stem cells. Furthermore, the fate decision of MSCs from different sources is regulated by distinct responses of cells to differentiation stimulations despite sharing a common CD49f+CD146+ immunophenotype.
Conclusions: We established an accurate, robust, transgene-free method for screening adipogenesis associated cell surface proteins. This provided a valuable tool to investigate MSC-specific markers. Additionally, we showed a possible crosstalk between CD49f and CD146 modulates the adipogenesis of MSCs.
Keywords: CD146; CD49f; high-throughput screening; light scatter gating; mesenchymal stem cell-specific markers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

