The Warburg Trap: A Novel Therapeutic Approach for Targeting Osteosarcoma
- PMID: 38201265
- PMCID: PMC10778102
- DOI: 10.3390/cells13010061
The Warburg Trap: A Novel Therapeutic Approach for Targeting Osteosarcoma
Abstract
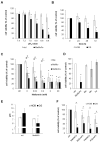
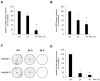
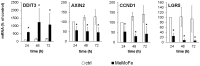
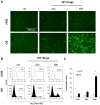
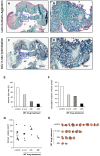
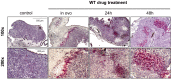
Although urgently needed, no significant improvements in osteosarcoma (OS) therapy have been achieved within the last decades. Here, we present a new therapeutic approach based on drug combinations consisting of mitochondrial complex I (MCI) inhibitors and ionophores that induce cancer cell-specific cell death based on a modulation of cellular energy metabolism and intracellular pH (pHi) named the Warburg Trap (WT). The effects of several drug combinations on intracellular pH, cell viability, colony-forming capacity and expression of WNT-target genes were analysed using OS cell lines and primary human osteoblasts (HOB). Tumour take rates and tumour volumes were analysed in vivo using a chicken chorioallantoic membrane assay (CAM). Several WT drug combinations induced the intracellular acidification and apoptotic cell death in OS cells, whereas HOBs tolerated the treatment. A significant inhibition of the colony-forming ability of OS cells and downregulation of WNT-target genes suggest that cancer stem cells (CSCs) are also targeted by the WT approach. In vivo, we observed a significant reduction in the tumour take rates in response to WT drug treatment. Our data suggest that the Warburg Trap is a promising approach for the development of a novel and effective OS therapy to replace or supplement the current OS chemotherapy.
Keywords: Warburg Trap; apoptosis; cancer stem cells; chemotherapy; osteosarcoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Gelderblom H., Jinks R.C., Sydes M., Bramwell V.H., van Glabbeke M., Grimer R.J., Hogendoorn P.C., McTiernan A., Lewis I.J., Nooij M.A., et al. Survival after recurrent osteosarcoma: Data from 3 European Osteosarcoma Intergroup (EOI) randomized controlled trials. Eur. J. Cancer. 2011;47:895–902. doi: 10.1016/j.ejca.2010.11.036. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

