Viral Targeting of Importin Alpha-Mediated Nuclear Import to Block Innate Immunity
- PMID: 38201275
- PMCID: PMC10778312
- DOI: 10.3390/cells13010071
Viral Targeting of Importin Alpha-Mediated Nuclear Import to Block Innate Immunity
Abstract
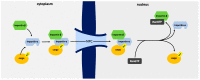
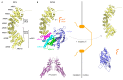
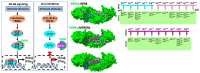
Cellular nucleocytoplasmic trafficking is mediated by the importin family of nuclear transport proteins. The well-characterized importin alpha (IMPA) and importin beta (IMPB) nuclear import pathway plays a crucial role in the innate immune response to viral infection by mediating the nuclear import of transcription factors such as IRF3, NFκB, and STAT1. The nuclear transport of these transcription factors ultimately leads to the upregulation of a wide range of antiviral genes, including IFN and IFN-stimulated genes (ISGs). To replicate efficiently in cells, viruses have developed mechanisms to block these signaling pathways. One strategy to evade host innate immune responses involves blocking the nuclear import of host antiviral transcription factors. By binding IMPA proteins, these viral proteins prevent the nuclear transport of key transcription factors and suppress the induction of antiviral gene expression. In this review, we describe examples of proteins encoded by viruses from several different families that utilize such a competitive inhibition strategy to suppress the induction of antiviral gene expression.
Keywords: African swine fever virus; Ebola virus; coronavirus; flavivirus; hantavirus; hepatitis B virus; human immunodeficiency virus-1; immune evasion; importin alpha; innate immunity; interferon; vaccinia virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Milles S., Mercadante D., Aramburu I.V., Jensen M.R., Banterle N., Koehler C., Tyagi S., Clarke J., Shammas S.L., Blackledge M., et al. Plasticity of an ultrafast interaction between nucleoporins and nuclear transport receptors. Cell. 2015;163:734–745. doi: 10.1016/j.cell.2015.09.047. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

