Modulation of Suppressive Activity and Proliferation of Human Regulatory T Cells by Splice-Switching Oligonucleotides Targeting FoxP3 Pre-mRNA
- PMID: 38201281
- PMCID: PMC10777989
- DOI: 10.3390/cells13010077
Modulation of Suppressive Activity and Proliferation of Human Regulatory T Cells by Splice-Switching Oligonucleotides Targeting FoxP3 Pre-mRNA
Abstract
The maturation, development, and function of regulatory T cells (Tregs) are under the control of the crucial transcription factor Forkhead Box Protein 3 (FoxP3). Through alternative splicing, the human FoxP3 gene produces four different splice variants: a full-length variant (FL) and truncated variants with deletions of each of exons 2 (∆2 variant) or 7 (∆7 variant) or a deletion of both exons (∆2∆7 variant). Their involvement in the biology of Tregs as well as their association with autoimmune diseases remains to be clarified. The aim of this work was to induce a single FoxP3 splice variant in human Tregs by splice switching oligonucleotides and to monitor their phenotype and proliferative and suppressive activity. We demonstrated that Tregs from peripheral blood from patients with multiple sclerosis preferentially expressed truncated splice variants, while the FL variant was the major variant in healthy donors. Tregs with induced expression of truncated FoxP3 splice variants demonstrated lower suppressive activity than those expressing FL variants. Reduced suppression was associated with the decreased expression of Treg-associated suppressive surface molecules and the production of cytokines. The deletion of exons 2 and/or 7 also reduced the cell proliferation rate. The results of this study show an association between FoxP3 splice variants and Treg function and proliferation. The modulation of Treg suppressive activity by the induction of the FoxP3 FL variant can become a promising strategy for regenerative immunotherapy.
Keywords: FoxP3; alternative splicing; multiple sclerosis; regulatory T cells; splicing-switching oligonucleotides; suppressive activity.
Conflict of interest statement
All authors declare that no conflict of interest exist.
Figures


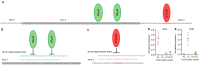


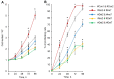







Similar articles
-
Induction of FoxP3 Pre-mRNA Alternative Splicing to Enhance the Suppressive Activity of Regulatory T Cells from Amyotrophic Lateral Sclerosis Patients.Biomedicines. 2024 May 7;12(5):1022. doi: 10.3390/biomedicines12051022. Biomedicines. 2024. PMID: 38790984 Free PMC article.
-
Malignant Tregs express low molecular splice forms of FOXP3 in Sézary syndrome.Leukemia. 2008 Dec;22(12):2230-9. doi: 10.1038/leu.2008.224. Epub 2008 Sep 4. Leukemia. 2008. PMID: 18769452
-
Direct targeting of FOXP3 in Tregs with AZD8701, a novel antisense oligonucleotide to relieve immunosuppression in cancer.J Immunother Cancer. 2022 Apr;10(4):e003892. doi: 10.1136/jitc-2021-003892. J Immunother Cancer. 2022. PMID: 35387780 Free PMC article. Clinical Trial.
-
A splice of life: the discovery, function, and clinical implications of FOXP3 isoforms in autoimmune disease.Int Immunol. 2024 Dec 26;37(2):83-90. doi: 10.1093/intimm/dxae049. Int Immunol. 2024. PMID: 39136284 Review.
-
Meta-Analysis of Alterations in Regulatory T Cells' Frequency and Suppressive Capacity in Patients with Vitiligo.J Immunol Res. 2022 Sep 16;2022:6952299. doi: 10.1155/2022/6952299. eCollection 2022. J Immunol Res. 2022. PMID: 36164321 Free PMC article. Review.
Cited by
-
Many Faces of Regulatory T Cells: Heterogeneity or Plasticity?Cells. 2024 Jun 1;13(11):959. doi: 10.3390/cells13110959. Cells. 2024. PMID: 38891091 Free PMC article. Review.
-
Association of Telomere Length in T Lymphocytes, B Lymphocytes, NK Cells and Monocytes with Different Forms of Age-Related Macular Degeneration.Biomedicines. 2024 Aug 19;12(8):1893. doi: 10.3390/biomedicines12081893. Biomedicines. 2024. PMID: 39200358 Free PMC article.
-
Induction of FoxP3 Pre-mRNA Alternative Splicing to Enhance the Suppressive Activity of Regulatory T Cells from Amyotrophic Lateral Sclerosis Patients.Biomedicines. 2024 May 7;12(5):1022. doi: 10.3390/biomedicines12051022. Biomedicines. 2024. PMID: 38790984 Free PMC article.
References
-
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. doi: 10.4049/jimmunol.155.3.1151. - DOI - PubMed
-
- Lifshitz G.V., Zhdanov D.D., Lokhonina A.V., Eliseeva D.D., Lyssuck E.Y., Zavalishin I.A., Bykovskaia S.N. Ex vivo expanded regulatory T cells CD4 + CD25 + FoxP3 + CD127 Low develop strong immunosuppressive activity in patients with remitting-relapsing multiple sclerosis. Autoimmunity. 2016;49:388–396. doi: 10.1080/08916934.2016.1199020. - DOI - PubMed
-
- Christodoulou M.I., Kapsogeorgou E.K., Moutsopoulos N.M., Moutsopoulos H.M. Foxp3+ T-regulatory cells in Sjogren’s syndrome: Correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. Am. J. Pathol. 2008;173:1389–1396. doi: 10.2353/ajpath.2008.080246. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

