Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells
- PMID: 38201302
- PMCID: PMC10777970
- DOI: 10.3390/cells13010096
Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells
Abstract
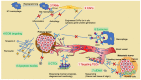
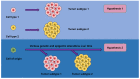
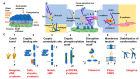
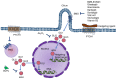
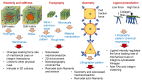
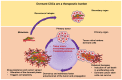
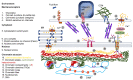
Extracellular biophysical properties have particular implications for a wide spectrum of cellular behaviors and functions, including growth, motility, differentiation, apoptosis, gene expression, cell-matrix and cell-cell adhesion, and signal transduction including mechanotransduction. Cells not only react to unambiguously mechanical cues from the extracellular matrix (ECM), but can occasionally manipulate the mechanical features of the matrix in parallel with biological characteristics, thus interfering with downstream matrix-based cues in both physiological and pathological processes. Bidirectional interactions between cells and (bio)materials in vitro can alter cell phenotype and mechanotransduction, as well as ECM structure, intentionally or unintentionally. Interactions between cell and matrix mechanics in vivo are of particular importance in a variety of diseases, including primarily cancer. Stiffness values between normal and cancerous tissue can range between 500 Pa (soft) and 48 kPa (stiff), respectively. Even the shear flow can increase from 0.1-1 dyn/cm2 (normal tissue) to 1-10 dyn/cm2 (cancerous tissue). There are currently many new areas of activity in tumor research on various biological length scales, which are highlighted in this review. Moreover, the complexity of interactions between ECM and cancer cells is reduced to common features of different tumors and the characteristics are highlighted to identify the main pathways of interaction. This all contributes to the standardization of mechanotransduction models and approaches, which, ultimately, increases the understanding of the complex interaction. Finally, both the in vitro and in vivo effects of this mechanics-biology pairing have key insights and implications for clinical practice in tumor treatment and, consequently, clinical translation.
Keywords: EMT; cancer cells; cancer-associated fibroblasts (CAFs); cell mechanics; extracellular matrix (ECM) remodeling; fibronectin; immune cells; mechanosensing; plasticity.
Conflict of interest statement
The author declares no conflicts of interest.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

