An Approach to Intersectionally Target Mature Enteroendocrine Cells in the Small Intestine of Mice
- PMID: 38201306
- PMCID: PMC10778503
- DOI: 10.3390/cells13010102
An Approach to Intersectionally Target Mature Enteroendocrine Cells in the Small Intestine of Mice
Abstract
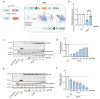
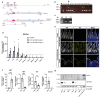
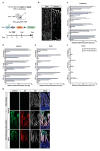
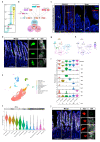
Enteroendocrine cells (EECs) constitute only a small proportion of Villin-1 (Vil1)-expressing intestinal epithelial cells (IECs) of the gastrointestinal tract; yet, in sum, they build the largest endocrine organ of the body, with each of them storing and releasing a distinct set of peptides for the control of feeding behavior, glucose metabolism, and gastrointestinal motility. Like all IEC types, EECs are continuously renewed from intestinal stem cells in the crypt base and terminally differentiate into mature subtypes while moving up the crypt-villus axis. Interestingly, EECs adjust their hormonal secretion according to their migration state as EECs receive altering differentiation signals along the crypt-villus axis and thus undergo functional readaptation. Cell-specific targeting of mature EEC subtypes by specific promoters is challenging because the expression of EEC-derived peptides and their precursors is not limited to EECs but are also found in other organs, such as the brain (e.g., Cck and Sst) as well as in the pancreas (e.g., Sst and Gcg). Here, we describe an intersectional genetic approach that enables cell type-specific targeting of functionally distinct EEC subtypes by combining a newly generated Dre-recombinase expressing mouse line (Vil1-2A-DD-Dre) with multiple existing Cre-recombinase mice and mouse strains with rox and loxP sites flanked stop cassettes for transgene expression. We found that transgene expression in triple-transgenic mice is highly specific in I but not D and L cells in the terminal villi of the small intestine. The targeting of EECs only in terminal villi is due to the integration of a defective 2A separating peptide that, combined with low EEC intrinsic Vil1 expression, restricts our Vil1-2A-DD-Dre mouse line and the intersectional genetic approach described here only applicable for the investigation of mature EEC subpopulations.
Keywords: Cck-expressing I cell; Cre/loxP; Dre/rox; EEC; Gcg-expressing L cell; Sst-expressing D cell; enteroendocrine cells of small intestine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

