Plasma Membrane Blebbing Is Controlled by Subcellular Distribution of Vimentin Intermediate Filaments
- PMID: 38201309
- PMCID: PMC10778383
- DOI: 10.3390/cells13010105
Plasma Membrane Blebbing Is Controlled by Subcellular Distribution of Vimentin Intermediate Filaments
Abstract
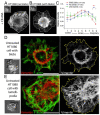
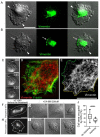
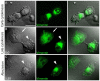
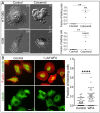
The formation of specific cellular protrusions, plasma membrane blebs, underlies the amoeboid mode of cell motility, which is characteristic for free-living amoebae and leukocytes, and can also be adopted by stem and tumor cells to bypass unfavorable migration conditions and thus facilitate their long-distance migration. Not all cells are equally prone to bleb formation. We have previously shown that membrane blebbing can be experimentally induced in a subset of HT1080 fibrosarcoma cells, whereas other cells in the same culture under the same conditions retain non-blebbing mesenchymal morphology. Here we show that this heterogeneity is associated with the distribution of vimentin intermediate filaments (VIFs). Using different approaches to alter the VIF organization, we show that blebbing activity is biased toward cell edges lacking abundant VIFs, whereas the VIF-rich regions of the cell periphery exhibit low blebbing activity. This pattern is observed both in interphase fibroblasts, with and without experimentally induced blebbing, and during mitosis-associated blebbing. Moreover, the downregulation of vimentin expression or displacement of VIFs away from the cell periphery promotes blebbing even in cells resistant to bleb-inducing treatments. Thus, we reveal a new important function of VIFs in cell physiology that involves the regulation of non-apoptotic blebbing essential for amoeboid cell migration and mitosis.
Keywords: blebbing; cell cortex; mesenchymal-to-amoeboid transition; vimentin intermediate filaments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Impairment of Assembly of the Vimentin Intermediate Filaments Leads to Suppression of Formation and Maturation of Focal Contacts and Alteration of the Type of Cellular Protrusions.Biochemistry (Mosc). 2024 Jan;89(1):184-195. doi: 10.1134/S0006297924010127. Biochemistry (Mosc). 2024. PMID: 38467554
-
Transition from mesenchymal to bleb-based motility is predominantly exhibited by CD133-positive subpopulation of fibrosarcoma cells.Biol Cell. 2019 Oct;111(10):245-261. doi: 10.1111/boc.201800078. Epub 2019 Aug 22. Biol Cell. 2019. PMID: 31403697
-
Vimentin intermediate filaments and filamentous actin form unexpected interpenetrating networks that redefine the cell cortex.Proc Natl Acad Sci U S A. 2022 Mar 8;119(10):e2115217119. doi: 10.1073/pnas.2115217119. Epub 2022 Mar 2. Proc Natl Acad Sci U S A. 2022. PMID: 35235449 Free PMC article.
-
Cell blebbing novel therapeutic possibilities to counter metastasis.Clin Exp Metastasis. 2024 Dec;41(6):817-828. doi: 10.1007/s10585-024-10308-z. Epub 2024 Sep 2. Clin Exp Metastasis. 2024. PMID: 39222238 Free PMC article. Review.
-
Signaling Pathways and Protein-Protein Interaction of Vimentin in Invasive and Migration Cells: A Review.Cell Reprogram. 2022 Aug;24(4):165-174. doi: 10.1089/cell.2022.0025. Epub 2022 Jun 24. Cell Reprogram. 2022. PMID: 35749708 Review.
Cited by
-
Genetic Animal Models of Idiopathic Generalized Epilepsies: What Can We Learn from Them?Biomedicines. 2025 May 26;13(6):1301. doi: 10.3390/biomedicines13061301. Biomedicines. 2025. PMID: 40564020 Free PMC article. Review.
-
Three-dimensional cell culture conditions promoted the Mesenchymal-Amoeboid Transition in the Triple-Negative Breast Cancer cell line MDA-MB-231.Front Cell Dev Biol. 2024 Aug 2;12:1435708. doi: 10.3389/fcell.2024.1435708. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39156975 Free PMC article.
-
Potential and value of rescuing dying neurons.Neural Regen Res. 2026 Mar 1;21(3):1013-1022. doi: 10.4103/NRR.NRR-D-24-01134. Epub 2025 Apr 29. Neural Regen Res. 2026. PMID: 40313097 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

