Temporal Trend of the SARS-CoV-2 Omicron Variant and RSV in the Nasal Cavity and Accuracy of the Newly Developed Antigen-Detecting Rapid Diagnostic Test
- PMID: 38201428
- PMCID: PMC10802845
- DOI: 10.3390/diagnostics14010119
Temporal Trend of the SARS-CoV-2 Omicron Variant and RSV in the Nasal Cavity and Accuracy of the Newly Developed Antigen-Detecting Rapid Diagnostic Test
Abstract
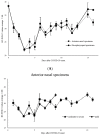
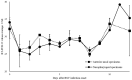
The aim of this work is to analyze the viral titers of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and respiratory syncytial virus (RSV) at the anterior nasal site (ANS) and nasopharyngeal site (NS), evaluate their virological dynamics, and validate the usefulness of a newly developed two-antigen-detecting rapid antigen diagnostic test (Ag-RDT) that simultaneously detects SARS-CoV-2 and RSV using clinical specimens. This study included 195 asymptomatic to severely ill patients. Overall, 668 specimens were collected simultaneously from the ANS and NS. The cycle threshold (Ct) values calculated from real-time polymerase chain reaction were used to analyze temporal changes in viral load and evaluate the sensitivity and specificity of the Ag-RDT. The mean Ct values for SARS-CoV-2-positive, ANS, and NS specimens were 28.8, 28.9, and 28.7, respectively. The mean Ct values for RSV-positive, ANS, and NS specimens were 28.7, 28.8, and 28.6, respectively. SARS-CoV-2 and RSV showed the same trend in viral load, although the viral load of NS was higher than that of ANS. The sensitivity and specificity of the newly developed Ag-RDT were excellent in specimens collected up to 10 days after the onset of SARS-CoV-2 infection and up to 6 days after the onset of RSV infection.
Keywords: RSV; RT-PCR; SARS-CoV-2; omicron; rapid diagnostic test; viral antigens.
Conflict of interest statement
Author Masaki Yoshihiro, Yuta Maehara, Shizuka Ito and Yasushi Ochiai was employed by the company Sekisui Medical Co., Ltd. The authors declare that this study received funding from Sekisui Medical Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures





References
-
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. [(accessed on 4 February 2023)]. Available online: https://covid19.who.int.
-
- World Health Organization Update on Omicron. [(accessed on 29 May 2023)]. Available online: https://www.who.int/news/item/28-11-2021-update-on-omicron.
-
- Puhach O., Adea K., Hulo N., Sattonnet P., Genecand C., Iten A., Jacquérioz F., Kaiser L., Vetter P., Eckerle I., et al. Infectious Viral Load in Unvaccinated and Vaccinated Individuals Infected with Ancestral, Delta or Omicron SARS-CoV-2. Nat. Med. 2022;28:1491–1500. doi: 10.1038/s41591-022-01816-0. - DOI - PubMed
-
- Tamura T., Ito J., Uriu K., Zahradnik J., Kida I., Anraku Y., Nasser H., Shofa M., Oda Y., Lytras S., et al. Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. Nat. Commun. 2023;14:2800. doi: 10.1038/s41467-023-38435-3. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

