The β-Secretase 1 Enzyme as a Novel Therapeutic Target for Prostate Cancer
- PMID: 38201438
- PMCID: PMC10778021
- DOI: 10.3390/cancers16010010
The β-Secretase 1 Enzyme as a Novel Therapeutic Target for Prostate Cancer
Abstract
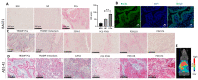
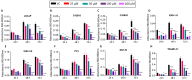
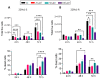
Recent studies have demonstrated the association of APP and Aβ with cancer, suggesting that BACE1 may play an important role in carcinogenesis. In the present study, we assessed BACE1's usefulness as a therapeutic target in prostate cancer (PCa). BACE1 expression was observed in human PCa tissue samples, patient-derived xenografts (PDX), human PCa xenograft tissue in nude mice, and transgenic adenocarcinoma of the mouse prostate (TRAMP) tissues by immunohistochemistry (IHC) analysis. Additionally, the downstream product of BACE1 activity, i.e., Aβ1-42 expression, was also observed in these PCa tissues by IHC as well as by PET imaging in TRAMP mice. Furthermore, BACE1 gene expression and activity was confirmed in several established PCa cell lines (LNCaP, C4-2B-enzalutamide sensitive [S], C4-2B-enzalutamide resistant [R], 22Rv1-S, 22Rv1-R, PC3, DU145, and TRAMP-C1) by real-time PCR and fluorometric assay, respectively. Treatment with a pharmacological inhibitor of BACE1 (MK-8931) strongly reduced the proliferation of PCa cells in in vitro and in vivo models, analyzed by multiple assays (MTT, clonogenic, and trypan blue exclusion assays and IHC). Cell cycle analyses revealed an increase in the sub-G1 population and a significant modulation in other cell cycle stages (G1/S/G2/M) following MK-8931 treatment. Most importantly, in vivo administration of MK-8931 intraperitoneal (30 mg/kg) strongly inhibited TRAMP-C1 allograft growth in immunocompetent C57BL/6 mice (approximately 81% decrease, p = 0.019). Furthermore, analysis of tumor tissue using the prostate cancer-specific pathway array revealed the alteration of several genes involved in PCa growth and progression including Forkhead O1 (FOXO1). All together, these findings suggest BACE1 as a novel therapeutic target in advanced PCa.
Keywords: BACE1; MK-8931; amyloid β peptide; prostate cancer.
Conflict of interest statement
G.D. is the founder of LiBiCo, which has no influence or contribution to the work presented in this manuscript. The authors declare no conflict of interest.
Figures




Similar articles
-
Activator of G protein signaling 3 modulates prostate tumor development and progression.Carcinogenesis. 2019 Dec 31;40(12):1504-1513. doi: 10.1093/carcin/bgz076. Carcinogenesis. 2019. PMID: 31215992 Free PMC article.
-
Host versus cell-dependent effects of β-arrestin 1 expression in prostate tumorigenesis.Carcinogenesis. 2021 May 28;42(5):772-783. doi: 10.1093/carcin/bgab021. Carcinogenesis. 2021. PMID: 33710266 Free PMC article.
-
Nintedanib antiangiogenic inhibitor effectiveness in delaying adenocarcinoma progression in Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP).J Biomed Sci. 2017 May 12;24(1):31. doi: 10.1186/s12929-017-0334-z. J Biomed Sci. 2017. PMID: 28499383 Free PMC article.
-
Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model: A good alternative to study PCa progression and chemoprevention approaches.Life Sci. 2019 Jan 15;217:141-147. doi: 10.1016/j.lfs.2018.12.002. Epub 2018 Dec 4. Life Sci. 2019. PMID: 30528182 Review.
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
Cited by
-
Identification of key targets and exploration of therapeutic molecular mechanisms of natural compound tangeretin in osteosarcoma.Discov Oncol. 2025 Jul 24;16(1):1401. doi: 10.1007/s12672-025-03221-8. Discov Oncol. 2025. PMID: 40705154 Free PMC article.
References
-
- Key Statistics for Prostate Cancer. [(accessed on 17 July 2022)]. Available online: https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics....
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

