Role of c-Src in Carcinogenesis and Drug Resistance
- PMID: 38201459
- PMCID: PMC10778207
- DOI: 10.3390/cancers16010032
Role of c-Src in Carcinogenesis and Drug Resistance
Abstract
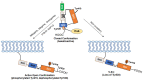
The aberrant transformation of normal cells into cancer cells, known as carcinogenesis, is a complex process involving numerous genetic and molecular alterations in response to innate and environmental stimuli. The Src family kinases (SFK) are key components of signaling pathways implicated in carcinogenesis, with c-Src and its oncogenic counterpart v-Src often playing a significant role. The discovery of c-Src represents a compelling narrative highlighting groundbreaking discoveries and valuable insights into the molecular mechanisms underlying carcinogenesis. Upon oncogenic activation, c-Src activates multiple downstream signaling pathways, including the PI3K-AKT pathway, the Ras-MAPK pathway, the JAK-STAT3 pathway, and the FAK/Paxillin pathway, which are important for cell proliferation, survival, migration, invasion, metastasis, and drug resistance. In this review, we delve into the discovery of c-Src and v-Src, the structure of c-Src, and the molecular mechanisms that activate c-Src. We also focus on the various signaling pathways that c-Src employs to promote oncogenesis and resistance to chemotherapy drugs as well as molecularly targeted agents.
Keywords: c-Src; carcinogenesis; drug resistance; signal transduction; v-Src.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

