Local Magnetic Hyperthermia and Systemic Gemcitabine/Paclitaxel Chemotherapy Triggers Neo-Angiogenesis in Orthotopic Pancreatic Tumors without Involvement of Auto/Paracrine Tumor Cell VEGF Signaling and Hypoxia
- PMID: 38201461
- PMCID: PMC10778317
- DOI: 10.3390/cancers16010033
Local Magnetic Hyperthermia and Systemic Gemcitabine/Paclitaxel Chemotherapy Triggers Neo-Angiogenesis in Orthotopic Pancreatic Tumors without Involvement of Auto/Paracrine Tumor Cell VEGF Signaling and Hypoxia
Abstract
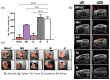
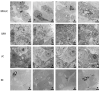
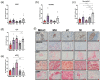
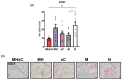
There is a growing interest in exploring the therapeutically mediated modulation of tumor vascularization of pancreatic cancer, which is known for its poorly perfused tumor microenvironment limiting the delivery of therapeutic agents to the tumor site. Here, we assessed how magnetic hyperthermia in combination with chemotherapy selectively affects growth, the vascular compartment of tumors, and the presence of tumor cells expressing key regulators of angiogenesis. To that purpose, a orthotopic PANC-1 (fluorescent human pancreatic adenocarcinoma) mouse tumor model (Rj:Athym-Foxn1nu/nu) was used. Magnetic hyperthermia was applied alone or in combination with systemic chemotherapy (gemcitabine 50 mg per kg body weight, nab-pacitaxel 30 mg/kg body weight) on days 1 and 7 following magnetic nanoparticle application (dose: 1 mg per 100 mm3 of tumor). We used ultrasound imaging, immunohistochemistry, multi-spectral optoacoustic tomography (MSOT), and hematology to assess the biological parameters mentioned above. We found that magnetic hyperthermia in combination with gemcitabine/paclitaxel chemotherapy was able to impact tumor growth (decreased volumes and Ki67 expression) and to trigger neo-angiogenesis (increased small vessel diameter) as a result of the therapeutically mediated cell damages/stress in tumors. The applied stressors activated specific pro-angiogenic mechanisms, which differed from those seen in hypoxic conditions involving HIF-1α, since (a) treated tumors showed a significant decrease of cells expressing VEGF, CD31, HIF-1α, and neuropilin-1; and (b) the relative tumor blood volume and oxygen level remained unchanged. Neo-angiogenesis seems to be the result of the activation of cell stress pathways, like MAPK pathways (high number of pERK-expressing tumor cells). In the long term, the combination of magnetic hyperthermia and chemotherapy could potentially be applied to transiently modulate tumor angiogenesis and to improve drug accessibility during oncologic therapies of pancreatic cancer.
Keywords: angiogenesis; chemotherapy; hypoxia; magnetic hyperthermia; orthotopic tumor model; pancreatic adenocarcinoma (PDAC).
Conflict of interest statement
The authors Paul Southern and Quentin A. Pankhurst were employed in part by the company Resonant Circuits Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Wang S., Zheng Y., Yang F., Zhu L., Zhu X.Q., Wang Z.F., Wu X.L., Zhou C.H., Yan J.Y., Hu B.Y., et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021;6:249. doi: 10.1038/s41392-021-00659-4. - DOI - PMC - PubMed
-
- Ganga A., Kim E.J., Mintzer G.L., Adriance W., Wang R., Cholankeril G., Balkrishnan R., Somasundar P.S. Disparities in primary pancreatic adenocarcinoma survival by Medicaid-status: A national population-based risk analysis. Eur. J. Surg. Oncol. 2023;49:1242–1249. doi: 10.1016/j.ejso.2023.02.002. - DOI - PubMed
-
- Iqbal M., Khawaja U.A., Soomro U., Rizvi S.A.A., Rizvi Z.H. Pancreatic adenocarcinoma in the elderly–recurrence and survival: A physician’s challenge. Adv. Cancer Biol.-Metastasis. 2023;7:100092. doi: 10.1016/j.adcanc.2023.100092. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

