Natural Anticancer Peptides from Marine Animal Species: Evidence from In Vitro Cell Model Systems
- PMID: 38201464
- PMCID: PMC10777987
- DOI: 10.3390/cancers16010036
Natural Anticancer Peptides from Marine Animal Species: Evidence from In Vitro Cell Model Systems
Abstract
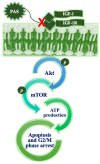
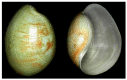
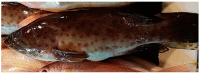
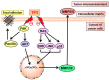
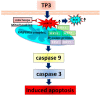
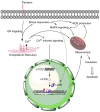
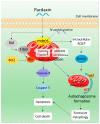
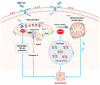
Anticancer peptides are short and structurally heterogeneous aminoacidic chains, which display selective cytotoxicity mostly against tumor cells, but not healthy cells, based on their different cell surface properties. Their anti-tumoral activity is carried out through interference with intracellular homeostasis, such as plasmalemma integrity, cell cycle control, enzymatic activities and mitochondrial functions, ultimately acting as angiogenesis-, drug resistance- and metastasis-inhibiting agents, immune stimulators, differentiation inducers and necrosis or extrinsic/intrinsic apoptosis promoters. The marine environment features an ever-growing level of biodiversity, and seas and oceans are poorly exploited mines in terms of natural products of biomedical interest. Adaptation processes to extreme and competitive environmental conditions led marine species to produce unique metabolites as a chemical strategy to allow inter-individual signalization and ensure survival against predators, infectious agents or UV radiation. These natural metabolites have found broad use in various applications in healthcare management, due to their anticancer, anti-angiogenic, anti-inflammatory and regeneration abilities. The aim of this review is to pick selected studies that report on the isolation of marine animal-derived peptides and the identification of their anticancer activity in in vitro cultures of cancer cells, and list them with respect to the taxonomical hierarchy of the source organism.
Keywords: Annelida; Arthropoda; Chordata; Cnidaria; Echinodermata; Mollusca; Porifera; anticancer peptides; marine drugs; tumor cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

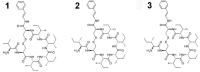

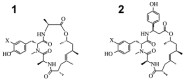
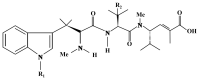




















References
-
- Rogers A.D., Appeltans W., Assis J., Balance L.T., Cury P., Duarte C., Favoretto F., Hynes L.A., Kumagai J.A., Lovelock C.E., et al. Discovering marine biodiversity in the 21st century. Adv. Mar. Biol. 2022;93:23–115. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

