Association of Clostridium butyricum Therapy Using the Live Bacterial Product CBM588 with the Survival of Patients with Lung Cancer Receiving Chemoimmunotherapy Combinations
- PMID: 38201474
- PMCID: PMC10778075
- DOI: 10.3390/cancers16010047
Association of Clostridium butyricum Therapy Using the Live Bacterial Product CBM588 with the Survival of Patients with Lung Cancer Receiving Chemoimmunotherapy Combinations
Abstract
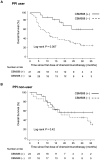
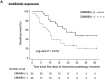
The gut microbiota has emerged as a key regulator of immune checkpoint inhibitor (ICI) efficacy. Therapeutic approaches aimed at manipulating the microbiota through targeted reconstitution to enhance cancer treatment outcomes have garnered considerable attention. A single live microbial biotherapeutic bacterium, Clostridium butyricum MIYAIRI 588 strain (CBM588), has been shown to enhance the effects of ICI monotherapy in patients with advanced lung cancer. However, whether CBM588 affects the outcomes of chemoimmunotherapy combinations in lung cancer remains unknown. We hypothesized that CBM588 augments the effect of chemoimmunotherapy combinations and restores diminished effectiveness in patients with non-small cell lung cancer (NSCLC) receiving dysbiosis-inducing drugs. To validate this hypothesis, we retrospectively analyzed 106 patients with stage IV or recurrent metastatic NSCLC consecutively treated with chemoimmunotherapy combinations. A survival analysis was performed employing univariate and multivariate Cox proportional hazard models with inverse probability of treatment weighting (IPTW) using propensity scores. Forty-five percent of patients received Clostridium butyricum therapy. CBM588 significantly extended overall survival in patients with NSCLC receiving chemoimmunotherapy. The favorable impact of CBM588 on the efficacy of chemoimmunotherapy combinations varied based on tumor-programmed cell death ligand 1 (PD-L1) expression. The survival benefit of CBM588 in the PD-L1 <1% cohort was higher than that in the PD-L1 1-49% and PD-L1 ≥ 50% cohorts. Furthermore, CBM588 was associated with improved overall survival in patients receiving proton pump inhibitors and/or antibiotics. CBM588-induced manipulation of the commensal microbiota holds the potential to enhance the efficacy of chemoimmunotherapy combinations, warranting further exploration of the synergy between CBM588 and immunotherapy.
Keywords: CBM588; Clostridium butyricum; antibiotics; dysbiosis; gut microbiome; immune checkpoint inhibitor; lung cancer; proton pump inhibitors.
Conflict of interest statement
Takuro Sakagami received research funding from Miyarisan Pharmaceutical Co., Ltd. The funder, Miyarisan Pharmaceutical Co., Ltd., was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. All authors declare no other competing interest.
Figures






References
-
- Derosa L., Routy B., Desilets A., Daillere R., Terrisse S., Kroemer G., Zitvogel L. Microbiota-centered interventions: The next breakthrough in immuno-oncology? Cancer Discov. 2021;11:2396–2412. doi: 10.1158/2159-8290.CD-21-0236. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

