Cyclooxygenase-2 Blockade Is Crucial to Restore Natural Killer Cell Activity before Anti-CTLA-4 Therapy against High-Grade Serous Ovarian Cancer
- PMID: 38201508
- PMCID: PMC10778357
- DOI: 10.3390/cancers16010080
Cyclooxygenase-2 Blockade Is Crucial to Restore Natural Killer Cell Activity before Anti-CTLA-4 Therapy against High-Grade Serous Ovarian Cancer
Abstract
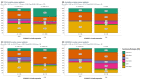
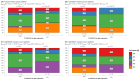
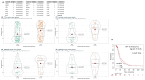
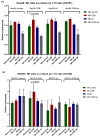
Chronic inflammation influences the tumor immune microenvironment (TIME) in high-grade serous ovarian cancer (HGSOC). Specifically, cyclooxygenase-2 (COX-2) overexpression promotes cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) expression. Notably, elevated COX-2 levels in the TIME have been associated with reduced response to anti-CTLA-4 immunotherapy. However, the precise impact of COX-2, encoded by PTGS2, on the immune profile remains unknown. To address this, we performed an integrated bioinformatics analysis using data from the HGSOC cohorts (TCGA-OV, n = 368; Australian cohort AOCS, n = 80; GSE26193, n = 62; and GSE30161, n = 45). Employing Gene Set Variation Analysis (GSVA), MIXTURE and Ecotyper cell deconvolution algorithms, we concluded that COX-2 was linked to immune cell ecosystems associated with shorter survival, cell dysfunction and lower NK cell effector cytotoxicity capacity. Next, we validated these results by characterizing circulating NK cells from HGSOC patients through flow cytometry and cytotoxic assays while undergoing COX-2 and CTLA-4 blockade. The blockade of COX-2 improved the cytotoxic capacity of NK cells against HGSOC cell lines. Our findings underscore the relevance of COX-2 in shaping the TIME and suggest its potential as a prognostic indicator and therapeutic target. Increased COX-2 expression may hamper the effectivity of immunotherapies that require NK cell effector function. These results provide a foundation for experimental validation and clinical trials investigating combined therapies targeting COX-2 and CTLA-4 in HGSOC.
Keywords: NK cells; cyclooxygenase-2; cytotoxic T-lymphocyte-associated protein-4; high-grade serious ovarian cancer; immunotherapy; tumor immune microenvironment.
Conflict of interest statement
The authors declare no conflict of interest related to this research.
Figures






References
-
- Cheng Z., Mirza H., Ennis D.P., Smith P., Morrill Gavarró L., Sokota C., Giannone G., Goranova T., Bradley T., Piskorz A., et al. The Genomic Landscape of Early-Stage Ovarian High-Grade Serous Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022;28:2911–2922. doi: 10.1158/1078-0432.CCR-21-1643. - DOI - PMC - PubMed
-
- Pinto M.P., Balmaceda C., Bravo M.L., Kato S., Villarroel A., Owen G.I., Roa J.C., Cuello M.A., Ibañez C. Patient inflammatory status and CD4+/CD8+ intraepithelial tumor lymphocyte infiltration are predictors of outcomes in high-grade serous ovarian cancer. Gynecol. Oncol. 2018;151:10–17. doi: 10.1016/j.ygyno.2018.07.025. - DOI - PubMed
-
- Knutson K.L., Maurer M.J., Preston C.C., Moysich K.B., Goergen K., Hawthorne K.M., Cunningham J.M., Odunsi K., Hartmann L.C., Kalli K.R., et al. Regulatory T cells, inherited variation, and clinical outcome in epithelial ovarian cancer. Cancer Immunol. Immunother. CII. 2015;64:1495–1504. doi: 10.1007/s00262-015-1753-x. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

