Synergistic Suppression of NF1 Malignant Peripheral Nerve Sheath Tumor Cell Growth in Culture and Orthotopic Xenografts by Combinational Treatment with Statin and Prodrug Farnesyltransferase Inhibitor PAMAM G4 Dendrimers
- PMID: 38201517
- PMCID: PMC10778372
- DOI: 10.3390/cancers16010089
Synergistic Suppression of NF1 Malignant Peripheral Nerve Sheath Tumor Cell Growth in Culture and Orthotopic Xenografts by Combinational Treatment with Statin and Prodrug Farnesyltransferase Inhibitor PAMAM G4 Dendrimers
Abstract
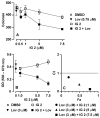
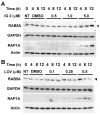
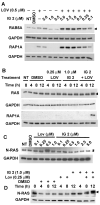
Neurofibromatosis type 1 (NF1) is a disorder in which RAS is constitutively activated due to the loss of the Ras-GTPase-activating activity of neurofibromin. RAS must be prenylated (i.e., farnesylated or geranylgeranylated) to traffic and function properly. Previous studies showed that the anti-growth properties of farnesyl monophosphate prodrug farnesyltransferase inhibitors (FTIs) on human NF1 malignant peripheral nerve sheath tumor (MPNST) cells are potentiated by co-treatment with lovastatin. Unfortunately, such prodrug FTIs have poor aqueous solubility. In this study, we synthesized a series of prodrug FTI polyamidoamine generation 4 (PAMAM G4) dendrimers that compete with farnesyl pyrophosphate for farnesyltransferase (Ftase) and assessed their effects on human NF1 MPNST S462TY cells. The prodrug 3-tert-butylfarnesyl monophosphate FTI-dendrimer (i.e., IG 2) exhibited improved aqueous solubility. Concentrations of IG 2 and lovastatin (as low as 0.1 μM) having little to no effect when used singularly synergistically suppressed cell proliferation, colony formation, and induced N-RAS, RAP1A, and RAB5A deprenylation when used in combination. Combinational treatment had no additive or synergistic effects on the proliferation/viability of immortalized normal rat Schwann cells, primary rat hepatocytes, or normal human mammary epithelial MCF10A cells. Combinational, but not singular, in vivo treatment markedly suppressed the growth of S462TY xenografts established in the sciatic nerves of immune-deficient mice. Hence, prodrug farnesyl monophosphate FTIs can be rendered water-soluble by conjugation to PAMAM G4 dendrimers and exhibit potent anti-tumor activity when combined with clinically achievable statin concentrations.
Keywords: MPNST; RAS prenylation; farnesyltransferase inhibitor; sciatic nerve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Induction of apoptosis in neurofibromatosis type 1 malignant peripheral nerve sheath tumor cell lines by a combination of novel farnesyl transferase inhibitors and lovastatin.J Pharmacol Exp Ther. 2008 Jul;326(1):1-11. doi: 10.1124/jpet.107.135830. Epub 2008 Mar 26. J Pharmacol Exp Ther. 2008. PMID: 18367665 Free PMC article.
-
Farnesyltransferase inhibitors block the neurofibromatosis type I (NF1) malignant phenotype.Cancer Res. 1995 Aug 15;55(16):3569-75. Cancer Res. 1995. PMID: 7627966
-
Brain lipid binding protein in axon-Schwann cell interactions and peripheral nerve tumorigenesis.Mol Cell Biol. 2003 Mar;23(6):2213-24. doi: 10.1128/MCB.23.6.2213-2224.2003. Mol Cell Biol. 2003. PMID: 12612091 Free PMC article.
-
New Model Systems and the Development of Targeted Therapies for the Treatment of Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors.Genes (Basel). 2020 Apr 28;11(5):477. doi: 10.3390/genes11050477. Genes (Basel). 2020. PMID: 32353955 Free PMC article. Review.
-
The promise of signal transduction in genetically driven sarcomas of the nerve.Exp Neurol. 2018 Jan;299(Pt B):317-325. doi: 10.1016/j.expneurol.2017.08.014. Epub 2017 Aug 30. Exp Neurol. 2018. PMID: 28859862 Review.
References
-
- Perry A., Roth K.A., Banerjee R., Fuller C.E., Gutmann D.H. NF1 deletions in S-100 protein-positive and negative cells of sporadic and neurofibromatosis 1 (NF1)-associated plexiform neurofibromas and malignant peripheral nerve sheath tumors. Am. J. Pathol. 2001;159:57–61. doi: 10.1016/S0002-9440(10)61673-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

