Extracellular Signal-Regulated Kinases: One Pathway, Multiple Fates
- PMID: 38201521
- PMCID: PMC10778234
- DOI: 10.3390/cancers16010095
Extracellular Signal-Regulated Kinases: One Pathway, Multiple Fates
Abstract
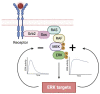
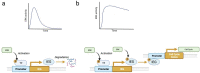
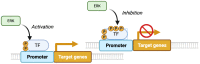
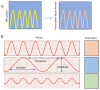
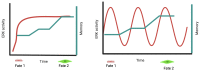
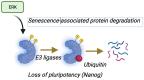
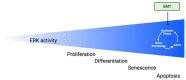
This comprehensive review delves into the multifaceted aspects of ERK signaling and the intricate mechanisms underlying distinct cellular fates. ERK1 and ERK2 (ERK) govern proliferation, transformation, epithelial-mesenchymal transition, differentiation, senescence, or cell death, contingent upon activation strength, duration, and context. The biochemical mechanisms underlying these outcomes are inadequately understood, shaped by signaling feedback and the spatial localization of ERK activation. Generally, ERK activation aligns with the Goldilocks principle in cell fate determination. Inadequate or excessive ERK activity hinders cell proliferation, while balanced activation promotes both cell proliferation and survival. Unraveling the intricacies of how the degree of ERK activation dictates cell fate requires deciphering mechanisms encompassing protein stability, transcription factors downstream of ERK, and the chromatin landscape.
Keywords: EMT; ERK; apoptosis; cell fate; cell proliferation; cell signaling; pluripotency; senescence.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Depletion of ERK2 but not ERK1 abrogates oncogenic Ras-induced senescence.Cell Signal. 2013 Dec;25(12):2540-7. doi: 10.1016/j.cellsig.2013.08.014. Epub 2013 Aug 30. Cell Signal. 2013. PMID: 23993963
-
ERK signalling: a master regulator of cell behaviour, life and fate.Nat Rev Mol Cell Biol. 2020 Oct;21(10):607-632. doi: 10.1038/s41580-020-0255-7. Epub 2020 Jun 23. Nat Rev Mol Cell Biol. 2020. PMID: 32576977 Review.
-
Small G proteins Rac1 and Ras regulate serine/threonine protein phosphatase 5 (PP5)·extracellular signal-regulated kinase (ERK) complexes involved in the feedback regulation of Raf1.J Biol Chem. 2014 Feb 14;289(7):4219-32. doi: 10.1074/jbc.M113.518514. Epub 2013 Dec 26. J Biol Chem. 2014. PMID: 24371145 Free PMC article.
-
TDAG51 is an ERK signaling target that opposes ERK-mediated HME16C mammary epithelial cell transformation.BMC Cancer. 2008 Jul 2;8:189. doi: 10.1186/1471-2407-8-189. BMC Cancer. 2008. PMID: 18597688 Free PMC article.
-
Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis.J Recept Signal Transduct Res. 2015;35(6):600-4. doi: 10.3109/10799893.2015.1030412. Epub 2015 Jun 22. J Recept Signal Transduct Res. 2015. PMID: 26096166 Review.
Cited by
-
Tumor dormancy and relapse: understanding the molecular mechanisms of cancer recurrence.Mil Med Res. 2025 Feb 11;12(1):7. doi: 10.1186/s40779-025-00595-2. Mil Med Res. 2025. PMID: 39934876 Free PMC article. Review.
-
Flavokawain A suppresses the malignant progression of neuroblastoma in vitro depending on inactivation of ERK/VEGF/MMPs signaling pathway.Discov Oncol. 2024 Nov 19;15(1):677. doi: 10.1007/s12672-024-01568-y. Discov Oncol. 2024. PMID: 39560824 Free PMC article.
-
Pentagalloyl Glucose from Bouea macrophylla Suppresses the Epithelial-Mesenchymal Transition and Synergizes the Doxorubicin-Induced Anticancer and Anti-Migration Effects in Triple-Negative Breast Cancer.Pharmaceuticals (Basel). 2024 Dec 20;17(12):1729. doi: 10.3390/ph17121729. Pharmaceuticals (Basel). 2024. PMID: 39770571 Free PMC article.
-
Metabolic and Regulatory Pathways Involved in the Anticancer Activity of Perillyl Alcohol: A Scoping Review of In Vitro Studies.Cancers (Basel). 2024 Nov 29;16(23):4003. doi: 10.3390/cancers16234003. Cancers (Basel). 2024. PMID: 39682189 Free PMC article.
-
Silencing PCCA Suppresses CRC Growth and Spread by Modulating EMT and M1 Macrophage Polarization.Int J Med Sci. 2025 Jan 1;22(1):87-100. doi: 10.7150/ijms.102046. eCollection 2025. Int J Med Sci. 2025. PMID: 39744168 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

