The Role of Receptor-Ligand Interaction in Somatostatin Signaling Pathways: Implications for Neuroendocrine Tumors
- PMID: 38201544
- PMCID: PMC10778465
- DOI: 10.3390/cancers16010116
The Role of Receptor-Ligand Interaction in Somatostatin Signaling Pathways: Implications for Neuroendocrine Tumors
Abstract
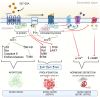
Neuroendocrine tumors (NETs) arise from neuroendocrine cells and manifest in diverse organs. Key players in their regulation are somatostatin and its receptors (SSTR1-SSTR5). Understanding receptor-ligand interactions and signaling pathways is vital for elucidating their role in tumor development and therapeutic potential. This review highlights SSTR characteristics, localization, and expression in tissues, impacting physiological functions. Mechanisms of somatostatin and synthetic analogue binding to SSTRs, their selectivity, and their affinity were analyzed. Upon activation, somatostatin initiates intricate intracellular signaling, involving cAMP, PLC, and MAP kinases and influencing growth, differentiation, survival, and hormone secretion in NETs. This review explores SSTR expression in different tumor types, examining receptor activation effects on cancer cells. SSTRs' significance as therapeutic targets is discussed. Additionally, somatostatin and analogues' role in hormone secretion regulation, tumor growth, and survival is emphasized, presenting relevant therapeutic examples. In conclusion, this review advances the knowledge of receptor-ligand interactions and signaling pathways in somatostatin receptors, with potential for improved neuroendocrine tumor treatments.
Keywords: SSTR characteristics; hormone secretion regulation; intracellular signaling; neuroendocrine tumors; receptor–ligand interactions; therapeutic targets; tumor development mechanisms.
Conflict of interest statement
The authors declare no conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
Epigenetic regulation of somatostatin and somatostatin receptors in neuroendocrine tumors and other types of cancer.Rev Endocr Metab Disord. 2021 Sep;22(3):495-510. doi: 10.1007/s11154-020-09607-z. Epub 2020 Oct 21. Rev Endocr Metab Disord. 2021. PMID: 33085037 Free PMC article. Review.
-
SSTR1 and SSTR5 subtypes are the dominant forms of somatostatin receptor in neuroendocrine tumors.Folia Histochem Cytobiol. 2010 Jan 1;48(1):142-7. doi: 10.2478/v10042-008-0103-7. Folia Histochem Cytobiol. 2010. PMID: 20529830
-
Somatostatin receptor biology in neuroendocrine and pituitary tumours: part 1--molecular pathways.J Cell Mol Med. 2010 Nov;14(11):2570-84. doi: 10.1111/j.1582-4934.2010.01125.x. J Cell Mol Med. 2010. PMID: 20629989 Free PMC article. Review.
-
Role of Somatostatin Signalling in Neuroendocrine Tumours.Int J Mol Sci. 2022 Jan 27;23(3):1447. doi: 10.3390/ijms23031447. Int J Mol Sci. 2022. PMID: 35163374 Free PMC article. Review.
-
Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy.Gastroenterology. 2010 Sep;139(3):742-53, 753.e1. doi: 10.1053/j.gastro.2010.07.002. Epub 2010 Jul 13. Gastroenterology. 2010. PMID: 20637207 Review.
Cited by
-
Crosstalk of TGF-β and somatostatin signaling in adenocarcinoma and neuroendocrine tumors of the pancreas: a brief review.Front Endocrinol (Lausanne). 2025 Mar 11;16:1511348. doi: 10.3389/fendo.2025.1511348. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40134804 Free PMC article. Review.
-
The Role of Somatostatin in the Gastrointestinal Tract.Biology (Basel). 2025 May 16;14(5):558. doi: 10.3390/biology14050558. Biology (Basel). 2025. PMID: 40427747 Free PMC article. Review.
-
"Cold" Somatostatin Analogs in Neuroendocrine Neoplasms: Decoding Mechanisms, Overcoming Resistance, and Shaping the Future of Therapy.Cells. 2025 Feb 9;14(4):245. doi: 10.3390/cells14040245. Cells. 2025. PMID: 39996718 Free PMC article. Review.
-
Assessing the diagnostic, prognostic, and therapeutic potential of the somatostatin/cortistatin system in glioblastoma.Cell Mol Life Sci. 2025 Apr 23;82(1):173. doi: 10.1007/s00018-025-05687-9. Cell Mol Life Sci. 2025. PMID: 40268793 Free PMC article.
-
Exosome: an overview on enhanced biogenesis by small molecules.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):6473-6508. doi: 10.1007/s00210-024-03762-9. Epub 2025 Jan 25. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39862264 Review.
References
-
- Yamada Y., Stoffel M., Espinosa R., 3rd, Xiang K.S., Seino M., Seino S., Le Beau M.M., Bell G.I. Human somatostatin receptor genes: Localization to human chromosomes 14, 17, and 22 and identification of simple tandem repeat polymorphisms. Genomics. 1993;15:449–452. doi: 10.1006/geno.1993.1088. - DOI - PubMed
-
- O’Carroll A.M., Raynor K., Lolait S.J., Reisine T. Characterization of cloned human somatostatin receptor SSTR5. Mol. Pharmacol. 1994;46:291–298. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

