Mogamulizumab Combined with Extracorporeal Photopheresis as a Novel Therapy in Erythrodermic Cutaneous T-cell Lymphoma
- PMID: 38201568
- PMCID: PMC10778082
- DOI: 10.3390/cancers16010141
Mogamulizumab Combined with Extracorporeal Photopheresis as a Novel Therapy in Erythrodermic Cutaneous T-cell Lymphoma
Abstract
Background: Primary cutaneous T-cell lymphomas (CTCLs) are rare lymphoproliferative malignancies characterized by significant morbidity and mortality in advanced disease stages. As curative approaches apart from allogeneic stem cell transplantation are lacking, establishing new treatment options, especially combination therapies, is crucial.
Methods: This retrospective study included 11 patients with SS or MF receiving therapy with mogamulizumab in combination with ECP from four European expert centers. The response rates in the skin and blood as well as treatment use and adverse events (AE) were described.
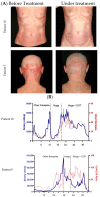
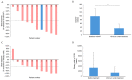
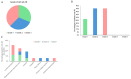
Results: 8/11 patients (73%) showed an overall response (OR) in the skin. The mean mSWAT decreased from 98.2 ± 40.8 to 34.6 ± 23.8. The overall response rate (ORR) in the blood was 64% with two complete responses. During combination therapy, the mean number of Sézary cells decreased from 3365.3 × 106/L before treatment to 1268.6 × 106/L. The mean minimum known period without progress was 7.2 months in the skin and 7.6 months in the blood. The most common AEs were mogamulizumab-associated rash (MAR) (45.5%), anemia (27.3%), lymphocytopenia (27.8%), and infusion related reaction (16.7%). No AE led to treatment discontinuation.
Conclusions: Our study presents the combination of mogamulizumab and ECP as an effective therapy in the blood and skin in CTCL with good tolerability, similar to mogamulizumab monotherapy.
Keywords: CTCL; Sézary syndrome; extracorporeal photopheresis; mogamulizumab; mycosis fungoides.
Conflict of interest statement
J.P.N. received travel and congress participation funding from TEVA and Novartis as well as consulting fees from TEVA, Almirall, Biogen, Novartis, Kyowa Kirin, Innate Pharma, Takeda and Actelion, UCB Pharma, and Recordati. S.M. received honoraria and travel funding from Kyowa Kirin. C.A. has performed consultancies for Takeda, Kyowa, Helsinn, Recordati, 4SC. Max Kappenstein works as consultant for the global management consultancy Bain & Company. N.N., I.H., M.B., and S.G. have no conflicts of interests to declare.
Figures
References
-
- Dippel E., Assaf C., Becker J.C., von Bergwelt-Baildon M., Bernreiter S., Cozzio A., Eich H.T., Elsayad K., Follmann M., Grabbe S., et al. S2k-Leitlinie—Kutane Lymphome (ICD10 C82-C86): Update 2021. J. Der Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG. 2022;20:537–555. doi: 10.1111/ddg.14706_g. - DOI - PubMed
-
- Kim Y.H., Willemze R., Pimpinelli N., Whittaker S., Olsen E.A., Ranki A., Dummer R., Hoppe R.T. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:479–484. doi: 10.1182/blood-2006-10-054601. - DOI - PubMed
-
- Swerdlow S.H., Campo E., Pileri S.A., Harris N.L., Stein H., Siebert R., Advani R., Ghielmini M., Salles G.A., Zelenetz A.D., et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. - DOI - PMC - PubMed
-
- Scarisbrick J.J., Prince H.M., Vermeer M.H., Quaglino P., Horwitz S., Porcu P., Stadler R., Wood G.S., Beylot-Barry M., Pham-Ledard A., et al. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:3766–3773. doi: 10.1200/JCO.2015.61.7142. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

