The Efficacy and Safety of Neoadjuvant Immunotherapy in Patients with Non-Small Cell Lung Cancer
- PMID: 38201583
- PMCID: PMC10778520
- DOI: 10.3390/cancers16010156
The Efficacy and Safety of Neoadjuvant Immunotherapy in Patients with Non-Small Cell Lung Cancer
Abstract
Background: After the success of immunotherapy in the treatment of advanced non-small cell lung cancer (NSCLC), the benefit of neoadjuvant chemoimmunotherapy was compared with chemotherapy for localized NSCLC in several trials. However, the available studies had variable study designs, and study cohorts had limited follow-up times. Therefore, we conducted a systematic review and meta-analysis to evaluate the benefit of adding immunotherapy to neoadjuvant chemotherapy in patients with localized NSCLC.
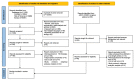
Methods: We conducted a systematic review using Pubmed, Web of Science, and Scopus databases for studies published until 5 December 2023. This protocol was registered in the PROSPERO database (Registration Number: CRD42023466337). We performed the meta-analyses with the generic inverse-variance method with a fixed effects model.
Results: Overall, 7 studies encompassing 2993 patients were included in the analyses. The use of neoadjuvant chemoimmunotherapy was associated with a 41% reduction in the risk of progression or death compared to neoadjuvant chemotherapy (HR: 0.59, 95% CI: 0.52-0.66, p < 0.0001) and a lower risk of death (HR: 0.67, 95% CI: 0.55-0.82, p < 0.0001). The neoadjuvant chemoimmunotherapy improved pCR rates compared to chemotherapy (21.8% vs. 3.8%, OR: 7.04, 95% CI: 5.23-9.47, p < 0.0001), while high-grade adverse events were higher with neoadjuvant chemoimmunotherapy (OR: 1.18, 95% CI: 1.02-1.36, p = 0.0300).
Conclusions: The available evidence demonstrates a statistically significant and clinically meaningful event-free survival benefit and possibly an overall survival benefit with neoadjuvant chemoimmunotherapy with a slight increase in high-grade toxicities.
Keywords: efficacy and safety; immune checkpoint inhibitors (ICIs); neoadjuvant chemoimmunotherapy; neoadjuvant immunotherapy; non-small cell lung cancer (NSCLC).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
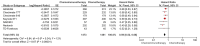
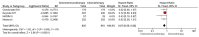


References
-
- Garon E.B., Hellmann M.D., Rizvi N.A., Carcereny E., Leighl N.B., Ahn M.J., Eder J.P., Balmanoukian A.S., Aggarwal C., Horn L., et al. Five-Year Overall Survival for Patients with Advanced Non-Small-Cell Lung Cancer Treated with Pembrolizumab: Results from the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019;37:2518–2527. doi: 10.1200/JCO.19.00934. - DOI - PMC - PubMed
-
- Pignon J.P., Tribodet H., Scagliotti G.V., Douillard J.Y., Shepherd F.A., Stephens R.J., Dunant A., Torri V., Rosell R., Seymour L., et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

