Comparative Study of the Effect of Radiation Delivered by Lutetium-177 or Actinium-225 on Anti-GD2 Chimeric Antigen Receptor T Cell Viability and Functions
- PMID: 38201618
- PMCID: PMC10778389
- DOI: 10.3390/cancers16010191
Comparative Study of the Effect of Radiation Delivered by Lutetium-177 or Actinium-225 on Anti-GD2 Chimeric Antigen Receptor T Cell Viability and Functions
Abstract
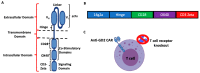
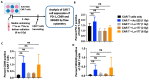
Chimeric antigen receptor (CAR) T cells have been relatively ineffective against solid tumors. Low-dose radiation which can be delivered to multiple sites of metastases by targeted radionuclide therapy (TRT) can elicit immunostimulatory effects. However, TRT has never been combined with CAR T cells against solid tumors in a clinical setting. This study investigated the effects of radiation delivered by Lutetium-177 (177Lu) and Actinium-225 (225Ac) on the viability and effector function of CAR T cells in vitro to evaluate the feasibility of such therapeutic combinations. After the irradiation of anti-GD2 CAR T cells with various doses of radiation delivered by 177Lu or 225Ac, their viability and cytotoxic activity against GD2-expressing human CHLA-20 neuroblastoma and melanoma M21 cells were determined by flow cytometry. The expression of the exhaustion marker PD-1, activation marker CD69 and the activating receptor NKG2D was measured on the irradiated anti-GD2 CAR T cells. Both 177Lu and 225Ac displayed a dose-dependent toxicity on anti-GD2 CAR T cells. However, radiation enhanced the cytotoxic activity of these CAR T cells against CHLA-20 and M21 irrespective of the dose tested and the type of radionuclide. No significant changes in the expression of PD-1, CD69 and NKG2D was noted on the CAR T cells following irradiation. Given a lower CAR T cell viability at equal doses and an enhancement of cytotoxic activity irrespective of the radionuclide type, 177Lu-based TRT may be preferred over 225Ac-based TRT when evaluating a potential synergism between these therapies in vivo against solid tumors.
Keywords: Actinium-225; Lutetium-177; chimeric antigen receptor; melanoma; neuroblastoma; targeted radionuclide therapy.
Conflict of interest statement
Q.H.S. is an inventor on patent applications related to this publication. MHF is an inventor on patent applications related to this publication. D.C.: None; C.P.K.: None; L.S.: None; A.S.: None; D.A.: None; J.C.E.: None; I.M.O.: None; R.H. received patent royalties from the Wisconsin Alumni Research Foundation; consulting fees from Archeus Technologies Inc. and Monopar Therapeutics. J.W.: is the cofounder of Archeus Technologies, which owns the licensing rights to NM600 and related technologies and holds stock in Archeus; B.P.B. received stock/stock options from Voximetry Inc.; K.S. received grant supports from Synthego and Spotlight Therapeutics; patent royalties from Wisconsin Alumni Research Foundation; honoraria from ISCT, is an inventor on patent applications related to this publication; Scientific Advisory Board Member for Notch Therapeutics and Andson Biotech. P.M.S. received support from the University of Wisconsin, Midwest Athletes For Childhood Cancer and the National Cancer Institute. C.M.C. received honoraria from Bayer, Nektar Therapeutics, Novartis, WiCell Research Institute, consulting fees from Elephas and is an inventor on patent applications related to this publication. Z.S.M. is a member of the Scientific Advisory Boards for Archeus Technologies, Seneca Therapeutics, and NorthStar Medical Isotopes; received royalties from patents held by the Wisconsin Alumni Research Foundation; received stock/stock options from Archeus Technologies Scientific Advisory board and Seneca Therapeutics Scientific Advisory Board; received research support from Point Biopharma, Telix Pharmaceuticals and XRD Therapeutics and is an inventor on patent applications related to this publication.
Figures


Similar articles
-
In vitro dose effect relationships of actinium-225- and lutetium-177-labeled PSMA-I&T.Eur J Nucl Med Mol Imaging. 2022 Sep;49(11):3627-3638. doi: 10.1007/s00259-022-05821-w. Epub 2022 May 12. Eur J Nucl Med Mol Imaging. 2022. PMID: 35556158 Free PMC article.
-
Eradication of Neuroblastoma by T Cells Redirected with an Optimized GD2-Specific Chimeric Antigen Receptor and Interleukin-15.Clin Cancer Res. 2019 May 1;25(9):2915-2924. doi: 10.1158/1078-0432.CCR-18-1811. Epub 2019 Jan 7. Clin Cancer Res. 2019. PMID: 30617136
-
CD171- and GD2-specific CAR-T cells potently target retinoblastoma cells in preclinical in vitro testing.BMC Cancer. 2019 Sep 9;19(1):895. doi: 10.1186/s12885-019-6131-1. BMC Cancer. 2019. PMID: 31500597 Free PMC article.
-
Paediatric Strategy Forum for medicinal product development of chimeric antigen receptor T-cells in children and adolescents with cancer: ACCELERATE in collaboration with the European Medicines Agency with participation of the Food and Drug Administration.Eur J Cancer. 2022 Jan;160:112-133. doi: 10.1016/j.ejca.2021.10.016. Epub 2021 Nov 25. Eur J Cancer. 2022. PMID: 34840026 Review.
-
Alpha and Beta Radiation for Theragnostics.PET Clin. 2024 Jul;19(3):307-323. doi: 10.1016/j.cpet.2024.03.006. Epub 2024 Apr 29. PET Clin. 2024. PMID: 38688775 Review.
Cited by
-
Unraveling Emerging Anal Cancer Clinical Biomarkers from Current Immuno-Oncogenomics Advances.Mol Diagn Ther. 2024 Mar;28(2):201-214. doi: 10.1007/s40291-023-00692-9. Epub 2024 Jan 24. Mol Diagn Ther. 2024. PMID: 38267771 Free PMC article. Review.
-
Cholesterol depletion suppresses thermal necrosis resistance by alleviating an increase in membrane fluidity.Sci Rep. 2025 Mar 24;15(1):10133. doi: 10.1038/s41598-025-92232-0. Sci Rep. 2025. PMID: 40128234 Free PMC article.
-
Platinum nanoparticles in cancer therapy: chemotherapeutic enhancement and ROS generation.Med Oncol. 2025 Jan 9;42(2):42. doi: 10.1007/s12032-024-02598-w. Med Oncol. 2025. PMID: 39789336 Review.
-
Low-dose radiation by radiopharmaceutical therapy enhances GD2 TRAC-CAR T cell efficacy in localized neuroblastoma.Sci Adv. 2025 Jun 6;11(23):eadu4417. doi: 10.1126/sciadv.adu4417. Epub 2025 Jun 4. Sci Adv. 2025. PMID: 40465728 Free PMC article.
-
Prospects of Synergy: Local Interventions and CAR T Cell Therapy in Solid Tumors.BioDrugs. 2024 Sep;38(5):611-637. doi: 10.1007/s40259-024-00669-y. Epub 2024 Jul 30. BioDrugs. 2024. PMID: 39080180 Free PMC article. Review.
References
-
- Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. - DOI - PMC - PubMed
-
- Abramson J.S., Palomba M.L., Gordon L.I., Lunning M.A., Wang M., Arnason J., Mehta A., Purev E., Maloney D.G., Andreadis C., et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

