A Ten-Year Real-Life Experience with Pazopanib in Uterine Leyomiosarcoma in Two High-Specialized Centers in Italy: Effectiveness and Safety
- PMID: 38201619
- PMCID: PMC10777896
- DOI: 10.3390/cancers16010192
A Ten-Year Real-Life Experience with Pazopanib in Uterine Leyomiosarcoma in Two High-Specialized Centers in Italy: Effectiveness and Safety
Abstract
Background: Uterine leiomyosarcoma (uLMS) is characterized by aggressive behavior associated with a high risk of relapse and mortality. Several therapeutic agents have been employed in the treatment of metastatic disease, with a poor objective response rate. Pazopanib, approved in 2012, is a multi-targeted, orally active small molecule that exerts its effects by inhibiting several tyrosine kinases. To date, poor research on real-life data has been conducted. We aimed to assess the effectiveness and safety of the drug in everyday clinical practice.
Methods: We present results of multicenter retrospective data on 38 patients with heavily pretreated metastatic uLMS who underwent oral pazopanib during their therapeutic journey.
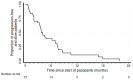
Results: At a median follow-up of 8.6 months, the disease control rate was 55.2%, with 17% partial responses and 15 patients (39.5%) with stable disease. At a median follow-up of 8.6 months, median progression-free survival was 4 months, and median overall survival was 19.8 months. The most common grade 3 adverse events (AEs) drug-related were hepatic toxicities, diarrhea, hypertension, nausea, and vomiting (all of them with an incidence of 5% considering the whole study cohort). No grade 4 AEs occurred.
Conclusions: Pazopanib in everyday clinical practice is safe and shows a good disease control rate with prolonged survival.
Keywords: effectiveness; pazopanib; safety; survival; uterine leyomiosarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ferrandina G., Aristei C., Biondetti P.R., Cananzi F.C.M., Casali P., Ciccarone F., Colombo N., Comandone A., Corvo’ R., De Iaco P., et al. Italian consensus conference on management of uterine sarcomas on behalf of S.I.G.O. (Societa’ italiana di Ginecologia E Ostetricia) Eur. J. Cancer. 2020;139:149–168. doi: 10.1016/j.ejca.2020.08.016. - DOI - PubMed
-
- Giuntoli R.L., 2nd, Metzinger D.S., DiMarco C.S., Cha S.S., Sloan J.A., Keeney G.L., Gostout B.S. Retrospective review of 208 patients with leiomyosarcoma of the uterus: Prognostic indicators, surgical management, and adjuvant therapy. Gynecol. Oncol. 2003;89:460–469. doi: 10.1016/S0090-8258(03)00137-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources