Limited Effects of Class II Transactivator-Based Immunotherapy in Murine and Human Glioblastoma
- PMID: 38201622
- PMCID: PMC10778432
- DOI: 10.3390/cancers16010193
Limited Effects of Class II Transactivator-Based Immunotherapy in Murine and Human Glioblastoma
Abstract
Background: The major histocompatibility complex type II is downregulated in glioblastoma (GB) due to the silencing of the major transcriptional regulator class II transactivator (CIITA). We investigated the pro-immunogenic potential of CIITA overexpression in mouse and human GB.
Methods: The intracerebral growth of wildtype GL261-WT cells was assessed following contralateral injection of GL261-CIITA cells or flank injections with GL261-WT or GL261-CIITA cells. Splenocytes obtained from mice implanted intracerebrally with GL261-WT, GL261-CIITA cells or phosphate buffered saline (PBS) were transferred to other mice and subsequently implanted intracerebrally with GL261-WT. Human GB cells and (syngeneic) GB-infiltrating immune cells were isolated from surgical samples and co-cultured with GB cells expressing CIITA or not, followed by RT-qPCR assessment of the expression of key immune regulators.
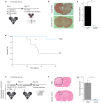
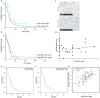
Results: Intracerebral vaccination of GL261-CIITA significantly reduced the subsequent growth of GL261-WT cells implanted contralaterally. Vaccination with GL261-WT or -CIITA subcutaneously, however, equivalently retarded the intracerebral growth of GL261 cells. Adoptive cell transfer experiments showed a similar antitumor potential of lymphocytes harvested from mice implanted intracerebrally with GL261-WT or -CIITA. Human GB-infiltrating myeloid cells and lymphocytes were not activated when cultured with CIITA-expressing GB cells. Tumor-infiltrating NK cells remained mostly inactivated when in co-culture with GB cells, regardless of CIITA.
Conclusion: these results question the therapeutic potential of CIITA-mediated immunotherapy in glioblastoma.
Keywords: class II transactivator; glioblastoma; natural killer cells; tumor microenvironment; tumor-associated macrophages; tumor-infiltrating lymphocytes.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Adenoviral delivery of the CIITA transgene induces T-cell-mediated killing in glioblastoma organoids.Mol Oncol. 2025 Mar;19(3):682-697. doi: 10.1002/1878-0261.13750. Epub 2024 Nov 13. Mol Oncol. 2025. PMID: 39535369 Free PMC article.
-
Non-Immune-Mediated, p27-Associated, Growth Inhibition of Glioblastoma by Class-II-Transactivator (CIITA).Cells. 2024 Nov 14;13(22):1883. doi: 10.3390/cells13221883. Cells. 2024. PMID: 39594630 Free PMC article.
-
Protective anti-tumor vaccination against glioblastoma expressing the MHC class II transactivator CIITA.Front Immunol. 2023 Mar 13;14:1133177. doi: 10.3389/fimmu.2023.1133177. eCollection 2023. Front Immunol. 2023. PMID: 36993983 Free PMC article.
-
CIITA-Driven MHC Class II Expressing Tumor Cells as Antigen Presenting Cell Performers: Toward the Construction of an Optimal Anti-tumor Vaccine.Front Immunol. 2019 Jul 30;10:1806. doi: 10.3389/fimmu.2019.01806. eCollection 2019. Front Immunol. 2019. PMID: 31417570 Free PMC article. Review.
-
CAR-Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors Into Precision Tools for Cancer Immunotherapy.Front Immunol. 2019 Nov 14;10:2683. doi: 10.3389/fimmu.2019.02683. eCollection 2019. Front Immunol. 2019. PMID: 31798595 Free PMC article. Review.
Cited by
-
Adenoviral delivery of the CIITA transgene induces T-cell-mediated killing in glioblastoma organoids.Mol Oncol. 2025 Mar;19(3):682-697. doi: 10.1002/1878-0261.13750. Epub 2024 Nov 13. Mol Oncol. 2025. PMID: 39535369 Free PMC article.
-
Non-Immune-Mediated, p27-Associated, Growth Inhibition of Glioblastoma by Class-II-Transactivator (CIITA).Cells. 2024 Nov 14;13(22):1883. doi: 10.3390/cells13221883. Cells. 2024. PMID: 39594630 Free PMC article.
References
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Stupp R., Taillibert S., Kanner A., Read W., Steinberg D.M., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma. JAMA. 2017;318:2306. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

