New N-Adducts of Thiadiazole and Thiazoline with Levoglucosenone and Evaluation of Their Significant Cytotoxic (Anti-Cancer) Activity
- PMID: 38201645
- PMCID: PMC10777969
- DOI: 10.3390/cancers16010216
New N-Adducts of Thiadiazole and Thiazoline with Levoglucosenone and Evaluation of Their Significant Cytotoxic (Anti-Cancer) Activity
Abstract
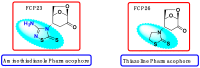
The conjugate N-adducts of thio-1,3,4-diazole and 2-thiazoline with levoglucosenone were synthesized via a stereoselective, base-catalyzed conjugate N-Michael addition to levoglucosenone at C-4. Structural assignments were established using 1H and 13C NMR analysis, and X-ray single-crystal analysis for one of the compounds. The biological properties of the novel compounds were tested on a cell model. Cytotoxicity was analyzed via colorimetric assay. Two distinct types of cell death, apoptosis and necrosis, were analyzed by determining the phosphatidylserine levels from the outer leaflet of the plasma membrane, caspase activation, and lactate dehydrogenase release. We also evaluated DNA damage using an alkaline comet assay. The level of oxidative stress was measured with a modified comet assay and an H2DCFDA probe. The thio-1,3,4-diazole adduct (FCP23) and the 2-thiazoline adduct (FCP26) exhibit similar cytotoxicity values for cancer cells (ovarian (A2780), breast (MCF-7), cervix (HeLa), colon (LoVo), and brain (MO59J and MO59K)), but their mechanism of action is drastically different. While FCP23 induces oxidative stress, DNA damage, and necrosis, FCP26 induces apoptosis through caspase activation.
Keywords: DNA damage; apoptosis; levoglucosenone; thiadiazole; thiazoline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


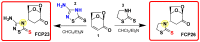






Similar articles
-
Thio-functionalized carbohydrate thiosemicarbazones and evaluation of their anticancer activity.Bioorg Med Chem Lett. 2017 Jun 15;27(12):2713-2720. doi: 10.1016/j.bmcl.2017.04.051. Epub 2017 Apr 21. Bioorg Med Chem Lett. 2017. PMID: 28506752
-
Effects of the chloro-s-triazine herbicide terbuthylazine on DNA integrity in human and mouse cells.Environ Sci Pollut Res Int. 2018 Jul;25(19):19065-19081. doi: 10.1007/s11356-018-2046-7. Epub 2018 May 2. Environ Sci Pollut Res Int. 2018. PMID: 29721798
-
Synthesis, structural characterizations and in vitro cytotoxic activities of new sulfonamide-based Schiff base derivatives.J Biomol Struct Dyn. 2023 Jul;41(10):4361-4367. doi: 10.1080/07391102.2022.2067237. Epub 2022 Apr 27. J Biomol Struct Dyn. 2023. PMID: 35477366
-
Design, synthesis, and biologic evaluation of novel chrysin derivatives as cytotoxic agents and caspase-3/7 activators.Drug Des Devel Ther. 2019 Jan 22;13:423-433. doi: 10.2147/DDDT.S189476. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 30774307 Free PMC article.
-
Genotoxicity of environmental agents assessed by the alkaline comet assay.Basic Clin Pharmacol Toxicol. 2005;96 Suppl 1:1-42. Basic Clin Pharmacol Toxicol. 2005. PMID: 15859009 Review.
References
-
- Sarnik J., Czubatka-Bienkowska A., Macieja A., Bielski R., Witczak Z.J., Poplawski T. The induction of oxidative stress in cervix carcinoma cells by levoglucosenone derived 4-S-salicyl derivative and (1-4)-S-thio-disaccharides. Part 4. Bioorg. Med. Chem. Lett. 2017;27:1215–1219. doi: 10.1016/j.bmcl.2017.01.064. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

