Preoperative 18F-FDG PET/CT in Patients with Presumed Localized Colon Cancer: A Prospective Study with Long-Term Follow-Up
- PMID: 38201660
- PMCID: PMC10777901
- DOI: 10.3390/cancers16010233
Preoperative 18F-FDG PET/CT in Patients with Presumed Localized Colon Cancer: A Prospective Study with Long-Term Follow-Up
Abstract
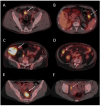
We analyzed whether preoperative 18F-FDG PET/CT adds to conventional primary staging in patients with presumed non-metastatic colonic cancer (CC). The prognostic role of 18F-FDG uptake in the primary tumor was evaluated after a mean follow-up of 15 years. Patients with a new diagnosis of presumed localized CC were prospectively enrolled and underwent presurgical 18F-FDG PET/CT. For each colon lesion, SUVmax, SUVpeak, TLG, and MTV were assessed and tested as prognostic factors. Forty-eight patients were included. Post-surgery pathology identified a total of 103 colon lesions, including 58 invasive adenocarcinomas, 4 in situ adenocarcinomas, 3 adenomas with high-grade dysplasia, and 38 adenomas with low-grade dysplasia. Per lesion sensitivity, specificity, positive (PPVs) and negative predictive values (NPVs) for colonic primary tumor detection were 78%, 97%, 98%, and 73% for conventional workup, and 94%, 87%, 92%, and 89% for 18F-FDG PET/CT. Only sensitivity was significantly different between 18F-FDG PET/CT and conventional workup. PET detected an additional ten pathological colonic lesions in seven patients. SUVmax, SUVpeak, and TLG showed significant differences between invasive adenocarcinomas, in situ adenocarcinomas, and high-grade dysplasia compared to low-grade dysplasia. There was a statistically significant difference between pT1-pT2 and pT3-pT4 adenocarcinomas. On patient-based analysis, sensitivity, specificity, PPV, and NPV for nodal staging were 22%, 84%, 44%, and 65% for CECT, and 33%, 90%, 67%, and 70% for 18F-FDG PET/CT, without a statistically significant difference. PET/CT also identified unknown metastatic spread and one synchronous lung cancer in four patients. Overall, 18F-FDG PETCT had an additional diagnostic value in 11 out of 48 patients (23%). 18F-FDG uptake of the primary tumor did not predict nodal or distant metastases. The difference in disease-free survival categorized by median SUVmax, SUVpeak, TLG, and MTV was not significant. Finally, preoperative 18F-FDG PET/CT is valuable in detecting potential colon lesions not visualized by conventional workups, especially in cases of incomplete colonoscopy. It effectively highlights distant metastases but exhibits limitations for N staging. Mainly due to the relatively small sample size, the quantitative analysis of 18F-FDG uptake in the primary tumor did not reveal any association with recurrence or disease-free survival, adding no significant prognostic information.
Keywords: 18F-FDG; PET/CT; carcinoma; colon; presurgical; staging; surgery.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Allemani C., Weir H.K., Carreira H., Harewood R., Spika D., Wang X.S., Bannon F., Ahn J.V., Johnson C.J., Bonaventure A., et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet Lond. Engl. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. - DOI - PMC - PubMed
-
- Argilés G., Tabernero J., Labianca R., Hochhauser D., Salazar R., Iveson T., Laurent-Puig P., Quirke P., Yoshino T., Taieb J., et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:1291–1305. doi: 10.1016/j.annonc.2020.06.022. - DOI - PubMed
-
- Gómez-España M.A., Gallego J., González-Flores E., Maurel J., Páez D., Sastre J., Aparicio J., Benavides M., Feliu J., Vera R. SEOM clinical guidelines for diagnosis and treatment of metastatic colorectal cancer. Clin. Transl. Oncol. 2019;21:46–54. doi: 10.1007/s12094-018-02002-w. - DOI - PMC - PubMed
-
- Gómez-España M.A., Gallego J., González-Flores E., Maurel J., Páez D., Sastre J., Aparicio J., Benavides M., Feliu J., Vera R. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. - PubMed
LinkOut - more resources
Full Text Sources

