Elucidating the Role of Optical Activity of Polymers in Protein-Polymer Interactions
- PMID: 38201730
- PMCID: PMC10781056
- DOI: 10.3390/polym16010065
Elucidating the Role of Optical Activity of Polymers in Protein-Polymer Interactions
Abstract
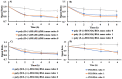
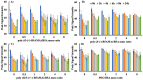
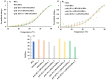
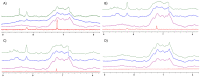
Proteins are biomolecules with potential applications in agriculture, food sciences, pharmaceutics, biotechnology, and drug delivery. Interactions of hydrophilic and biocompatible polymers with proteins may impart proteolytic stability, improving the therapeutic effects of biomolecules and also acting as excipients for the prolonged storage of proteins under harsh conditions. The interactions of hydrophilic and stealth polymers such as poly(ethylene glycol), poly(trehalose), and zwitterionic polymers with various proteins are well studied. This study evaluates the molecular interactions of hydrophilic and optically active poly(vitamin B5 analogous methacrylamide) (poly(B5AMA)) with model proteins by fluorescence spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and circular dichroism (CD) spectroscopy analysis. The optically active hydrophilic polymers prepared using chiral monomers of R-(+)- and S-(-)-B5AMA by the photo-iniferter reversible addition fragmentation chain transfer (RAFT) polymerization showed concentration-dependent weak interactions of the polymers with bovine serum albumin and lysozyme proteins. Poly(B5AMA) also exhibited a concentration-dependent protein stabilizing effect at elevated temperatures, and no effect of the stereoisomers of polymers on protein thermal stability was observed. NMR analysis, however, showed poly(B5AMA) stereoisomer-dependent changes in the secondary structure of proteins.
Keywords: antifouling; chiral materials; protein stabilizing; protein–polymer interactions.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Zhang P., Sun F., Tsao C., Liu S., Jain P., Sinclair A., Hung H.-C., Bai T., Wu K., Jiang S. Zwitterionic Gel Encapsulation Promotes Protein Stability, Enhances Pharmacokinetics, and Reduces Immunogenicity. Proc. Natl. Acad. Sci. USA. 2015;112:12046–12051. doi: 10.1073/pnas.1512465112. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

