Amurensin G Sensitized Cholangiocarcinoma to the Anti-Cancer Effect of Gemcitabine via the Downregulation of Cancer Stem-like Properties
- PMID: 38201903
- PMCID: PMC10780614
- DOI: 10.3390/nu16010073
Amurensin G Sensitized Cholangiocarcinoma to the Anti-Cancer Effect of Gemcitabine via the Downregulation of Cancer Stem-like Properties
Abstract
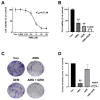
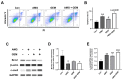
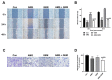
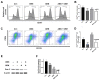
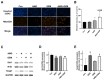
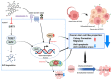
Cholangiocarcinoma (CCA) is a malignant biliary tract tumor with a high mortality rate and refractoriness to chemotherapy. Gemcitabine is an anti-cancer chemotherapeutic agent used for CCA, but the efficacy of gemcitabine in CCA treatment is limited, due to the acquisition of chemoresistance. The present study evaluated the chemosensitizing effects of Amurensin G (AMG), a natural sirtuin-1 inhibitor derived from Vitis amurensis, in the SNU-478 CCA cells. Treatment with AMG decreased the SNU-478 cell viability and the colony formation ability. Annexin V/ Propidium iodide staining showed that the AMG increased apoptotic death. In addition, AMG downregulated anti-apoptotic Bcl-2 expression, while upregulating pro-apoptotic cleaved caspase-3 expression. Treatment with AMG decreased the migratory ability of the cells in a wound healing assay and transwell migration assay. It was observed that AMG decreased the gemcitabine-induced increase in CD44highCD24highCD133high cell populations, and the expression of the Sox-2 protein was decreased by AMG treatment. Co-treatment of AMG with gemcitabine significantly enhanced the production of reactive oxygen species, as observed through mitochondrial superoxide staining, which might be associated with the downregulation of the Sirt1/Nrf2 pathway by AMG. These results indicate that AMG enhances the chemotherapeutic ability of gemcitabine by downregulating cancer stem-like properties in CCA cells. Hence, a combination therapy of AMG with gemcitabine may be an attractive therapeutic strategy for cholangiocarcinoma.
Keywords: amurensin G; cancer stem cell; chemoresistance; cholangiocarcinoma; gemcitabine.
Conflict of interest statement
The authors do not have any conflicts of interest to declare.
Figures
Similar articles
-
Cordycepin Enhanced Therapeutic Potential of Gemcitabine against Cholangiocarcinoma via Downregulating Cancer Stem-Like Properties.Biomol Ther (Seoul). 2024 May 1;32(3):369-378. doi: 10.4062/biomolther.2023.198. Epub 2024 Apr 9. Biomol Ther (Seoul). 2024. PMID: 38589021 Free PMC article.
-
Atorvastatin Augments Gemcitabine-Mediated Anti-Cancer Effects by Inhibiting Yes-Associated Protein in Human Cholangiocarcinoma Cells.Int J Mol Sci. 2020 Oct 14;21(20):7588. doi: 10.3390/ijms21207588. Int J Mol Sci. 2020. PMID: 33066548 Free PMC article.
-
Cannabidiol and Cannabigerol Inhibit Cholangiocarcinoma Growth In Vitro via Divergent Cell Death Pathways.Biomolecules. 2022 Jun 20;12(6):854. doi: 10.3390/biom12060854. Biomolecules. 2022. PMID: 35740979 Free PMC article.
-
Systemic therapy of cholangiocarcinoma: From chemotherapy to targeted therapies.Best Pract Res Clin Gastroenterol. 2015 Apr;29(2):345-53. doi: 10.1016/j.bpg.2015.01.002. Epub 2015 Feb 19. Best Pract Res Clin Gastroenterol. 2015. PMID: 25966433 Review.
-
Cholangiocarcinoma Therapeutics: An Update.Curr Cancer Drug Targets. 2021;21(6):457-475. doi: 10.2174/1568009621666210204152028. Curr Cancer Drug Targets. 2021. PMID: 33563168 Review.
Cited by
-
Causal relationship between immune cell phenotypes and risk of biliary tract cancer: evidence from Mendelian randomization analysis.Front Immunol. 2024 Jul 10;15:1430551. doi: 10.3389/fimmu.2024.1430551. eCollection 2024. Front Immunol. 2024. PMID: 39050844 Free PMC article.
References
-
- Banales J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., Cardinale V., Carpino G., Andersen J.B., Braconi C., et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

