Mediterranean Diet, Vitamin D, and Hypercaloric, Hyperproteic Oral Supplements for Treating Sarcopenia in Patients with Heart Failure-A Randomized Clinical Trial
- PMID: 38201939
- PMCID: PMC10781070
- DOI: 10.3390/nu16010110
Mediterranean Diet, Vitamin D, and Hypercaloric, Hyperproteic Oral Supplements for Treating Sarcopenia in Patients with Heart Failure-A Randomized Clinical Trial
Abstract
Background: Malnutrition and sarcopenia frequently affect patients with heart failure (HF), in which clinical outcomes and survival is decreased. Thus, appropriate nutritional screening and early nutrition support are highly recommended. Currently, nutritional support is not a standard of care in patients with HF, and the use of commercially available oral supplements (OSs) could provide an additional benefit to medical treatment in these patients.
Aim: To compare the effect of the Mediterranean diet in combination with hypercaloric, hyperproteic OS in patients with HF.
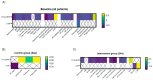
Patients and methods: An open label, controlled clinical study in which patients were randomly assigned to receive a Mediterranean diet (control group) vs. hypercaloric, hyperproteic OS (intervention group) for twenty-four weeks. Thirty-eight patients were included; epidemiological, clinical, anthropometric, ultrasound (muscle echography of the rectus femoris muscle of the quadriceps and abdominal adipose tissue), and biochemical evaluations were performed. All patients received additional supplementation with vitamin D.
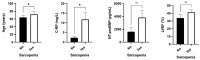
Results: Baseline malnutrition according to the GLIM criteria was observed in 30% of patients, while 65.8% presented with sarcopenia. Body cell mass, lean mass, and body mass increased in the intervention group (absolute increase of 0.5, p = 0.03, 1.2 kg, p = 0.03, and 0.1 kg, p = 0.03 respectively). In contrast, fat mass increased in the control group (4.5 kg, p = 0.05). According to the RF ultrasound, adipose tissue, muscle area, and circumference tended to decrease in the intervention group; it is probable that 24 weeks was too short a period of time for evaluating changes in muscle area or circumference, as previously observed in another group of patients. In contrast, functionality, determined by the up-and-go test, significantly improved in all patients (difference 12.6 s, p < 0.001), including the control (10 s improvement, p < 0.001) and the intervention group (improvement of 8.9 s, p < 0.001). Self-reported QoL significantly increased in all groups, from 68.7 ± 22.2 at baseline to 77.7 ± 18.7 (p = 0.01). When heart functionality was evaluated, LVEF increased in the whole cohort (38.7 ± 16.6 vs. 42.2 ± 8.9, p < 0.01); this increase was higher in the intervention group (34.2 ± 16.1 at baseline vs. 45.0% ± 17.0 after 24 weeks, p < 0.05). Serum values of NT-proBNP also significantly decreased in the whole cohort (p < 0.01), especially in the intervention group (p = 0.02). After adjusting by age and sex, nutritional support, baseline LVEF, NT-proBNP, and body composition parameters of functionality tests were not associated with mortality or new hospital admissions in this cohort.
Conclusion: Nutritional support with hypercaloric, hyperproteic OS, Mediterranean diet, and vitamin D supplementation were associated with decreased NT-proBNP and improvements in LVEF, functionality, and quality of life in patients with HF, despite a significant decrease in hospital admissions.
Keywords: LVEF; NT-proBNP; heart failure; oral supplements; sarcopenia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Differences in the Evaluation of Malnutrition and Body Composition Using Bioelectrical Impedance Analysis, Nutritional Ultrasound, and Dual-Energy X-ray Absorptiometry in Patients with Heart Failure.Nutrients. 2024 May 20;16(10):1535. doi: 10.3390/nu16101535. Nutrients. 2024. PMID: 38794773 Free PMC article.
-
Standard Hypercaloric, Hyperproteic vs. Leucine-Enriched Oral Supplements in Patients with Cancer-Induced Sarcopenia, a Randomized Clinical Trial.Nutrients. 2023 Jun 12;15(12):2726. doi: 10.3390/nu15122726. Nutrients. 2023. PMID: 37375630 Free PMC article. Clinical Trial.
-
Impact of Hydroxy-Methyl-Butyrate Supplementation on Malnourished Patients Assessed Using AI-Enhanced Ultrasound Imaging.J Cachexia Sarcopenia Muscle. 2025 Feb;16(1):e13700. doi: 10.1002/jcsm.13700. J Cachexia Sarcopenia Muscle. 2025. PMID: 39992793 Free PMC article.
-
Relevance of nutritional assessment and treatment to counteract cardiac cachexia and sarcopenia in chronic heart failure.Clin Nutr. 2021 Sep;40(9):5141-5155. doi: 10.1016/j.clnu.2021.07.027. Epub 2021 Jul 31. Clin Nutr. 2021. PMID: 34461588 Review.
-
A Narrative Review of the Diagnosis and Treatment of Sarcopenia and Malnutrition in Patients with Heart Failure.Nutrients. 2024 Aug 15;16(16):2717. doi: 10.3390/nu16162717. Nutrients. 2024. PMID: 39203852 Free PMC article. Review.
Cited by
-
Differences in the Evaluation of Malnutrition and Body Composition Using Bioelectrical Impedance Analysis, Nutritional Ultrasound, and Dual-Energy X-ray Absorptiometry in Patients with Heart Failure.Nutrients. 2024 May 20;16(10):1535. doi: 10.3390/nu16101535. Nutrients. 2024. PMID: 38794773 Free PMC article.
-
Nutrient Intake and Its Association with Appendicular Total Lean Mass and Muscle Function and Strength in Older Adults: A Population-Based Study.Nutrients. 2024 Feb 19;16(4):568. doi: 10.3390/nu16040568. Nutrients. 2024. PMID: 38398892 Free PMC article.
-
Current approach to the diagnosis of sarcopenia in cardiovascular diseases.Front Nutr. 2024 Nov 15;11:1422663. doi: 10.3389/fnut.2024.1422663. eCollection 2024. Front Nutr. 2024. PMID: 39619285 Free PMC article. Review.
-
Nutritional Support Reduces Circulating Cytokines in Patients with Heart Failure.Nutrients. 2024 May 27;16(11):1637. doi: 10.3390/nu16111637. Nutrients. 2024. PMID: 38892570 Free PMC article. Clinical Trial.
-
Improving the nutritional evaluation in head neck cancer patients using bioelectrical impedance analysis: Not only the phase angle matters.J Cachexia Sarcopenia Muscle. 2024 Dec;15(6):2426-2436. doi: 10.1002/jcsm.13577. Epub 2024 Oct 24. J Cachexia Sarcopenia Muscle. 2024. PMID: 39447215 Free PMC article.
References
-
- Herrera-Martínez A.D., León Idougourram S., Muñoz Jiménez C., Rodríguez-Alonso R., Alonso Echague R., Chica Palomino S., Sanz Sanz A., Manzano García G., Gálvez Moreno M.Á., Calañas Continente A., et al. Standard Hypercaloric, Hyperproteic vs. Leucine-Enriched Oral Supplements in Patients with Cancer-Induced Sarcopenia, a Randomized Clinical Trial. Nutrients. 2023;15:2726. doi: 10.3390/nu15122726. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

