Skin Anti-Aging Efficacy of Enzyme-Treated Supercritical Caviar Extract: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
- PMID: 38201966
- PMCID: PMC10780664
- DOI: 10.3390/nu16010137
Skin Anti-Aging Efficacy of Enzyme-Treated Supercritical Caviar Extract: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
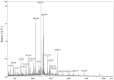
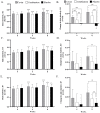
Oxidative stress in the skin, induced by an unhealthy lifestyle and exposure to UVB radiation, leads to skin aging, including reduced elasticity, formation of wrinkles, moisture loss, and inflammation. In a previous study, we revealed the photoaging effects of enzyme-treated caviar extract (CV) by regulating collagen and hyaluronic acid synthase, melanogenesis, anti-oxidant mechanisms, and inflammation in a UVB irradiation-induced mice model. HPLC and MALDI-TOF were performed to determine the effect of enzyme treatment on the free amino acid contents and peptide molecular weight in supercritical caviar extract. As results of the analysis, CV is mainly composed of low-molecular-weight peptides consisting of leucine, tyrosine, and phenylalanine. Based on our in vitro and in vivo study, we conducted a clinical trial to assess the skin anti-aging efficacy of CV. In this randomized, double-blind, placebo-controlled trial, we measured indicators related to elasticity, wrinkles, and skin hydration at 4 and 8 weeks after consumption of CV. The subjects were categorized into caviar, combination, and placebo groups. After 4 weeks, skin hydration, dermal hydration, and transepidermal water loss all showed significant improvement. Furthermore, after 8 weeks, skin elasticity indexes-R2 (total elasticity), R5 (net elasticity), and R7 (ratio of elastic recovery to total deformation)-exhibited significant increases. Improvement in wrinkle indicators (Rmax, Ra, and Rz) and the whitening indicator melanin pigment was also observed. This is the first report showing that CV has significant skin anti-aging efficacy on human skin. In conclusion, our study suggests that CV can be used as skin anti-aging nutraceuticals through positive effects on skin condition in clinical trials.
Keywords: caviar; skin anti-aging; skin elasticity; skin hydration; skin whitening; skin wrinkles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Lorenzetti A., Marotta F., Yadav H., Celep G., Minelli E., Carrera-Bastos P., Jain S., Polimeni A., Solimene U. Improving sperm quality and spermatogenesis through a bioactive marine compound: An experimental study. Acta Biomed. 2012;83:108–113. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

