Treatment of Relapsed or Refractory Diffuse Large B-Cell Lymphoma: New Approved Options
- PMID: 38202077
- PMCID: PMC10779497
- DOI: 10.3390/jcm13010070
Treatment of Relapsed or Refractory Diffuse Large B-Cell Lymphoma: New Approved Options
Abstract
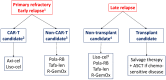
Overall, around 40% of patients with diffuse large B-cell lymphoma (DLBCL) have refractory disease or relapse after the first line of treatment. Until relatively recently, the prognosis of patients with relapsed or refractory DLBCL was very poor and treatment options were very limited. In recent years, several novel therapies have been approved that provide more effective options than conventional chemotherapy and that have manageable toxicity profiles. CAR-T cell therapy has become the new standard treatment for patients with refractory or early relapsed DLBCL, based on the positive results of the phase 3 ZUMA-7 and TRANSFORM clinical trials. This review addresses the role of CAR-T therapy and autologous stem cell transplantation in the treatment of these patients and other approved options for patients who are not candidates for transplant, such as the combinations of polatuzumab vedotin with bendamustine and rituximab, and tafasitamab with lenalidomide.
Keywords: CAR-T cell therapy; autologous stem cell transplantation; diffuse large-B cell lymphoma; polatuzumab vedotin; relapsed or refractory DLBCL; tafasitamab.
Conflict of interest statement
AMGS has received honoraria or consulting fees from Roche, BMS/Celgene, Janssen, Gilead/Kite, Takeda, Eusa Pharma, Sobi, Kyowa Kirin, Novartis, Incyte, Lilly, ADC Therapeutics America, Miltenyi, Ideogen, Abbvie.
Figures
References
-
- Cheson B.D., Fisher R.I., Barrington S.F., Cavalli F., Schwartz L.H., Zucca E., Lister T.A. Recommendations for initial evaluation, stag-ing, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. - DOI - PMC - PubMed
-
- Philip T., Guglielmi C., Hagenbeek A., Somers R., Van Der Lelie H., Bron D., Sonneveld P., Gisselbrecht C., Cahn J.-Y., Harousseau J.-L., et al. Autologous Bone Marrow Transplantation as Compared with Salvage Chemotherapy in Relapses of Chemotherapy-Sensitive Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. - DOI - PubMed
-
- Snowden J.A., Sánchez-Ortega I., Corbacioglu S., Basak G.W., Chabannon C., de la Camara R., Dolstra H., Duarte R.F., Glass B., Greco R., et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2022. Bone Marrow Transplant. 2022;57:1217–1239. doi: 10.1038/s41409-022-01691-w. - DOI - PMC - PubMed
-
- Redondo A.M., Valcárcel D., González-Rodríguez A.P., Suárez-Lledó M., Bello J.L., Canales M., Gayoso J., Colorado M., Jarque I., Del Campo R., et al. Bendamustine as part of condi-tioning of autologous stem cell transplantation in patients with aggressive lymphoma: A phase 2 study from the GELTAMO group. Br. J. Haematol. 2019;184:797–807. doi: 10.1111/bjh.15713. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

