Robotic Lateral Pelvic Lymph Node Dissection in Rectal Cancer: A Feasibility Study from a European Centre
- PMID: 38202097
- PMCID: PMC10779823
- DOI: 10.3390/jcm13010090
Robotic Lateral Pelvic Lymph Node Dissection in Rectal Cancer: A Feasibility Study from a European Centre
Abstract
Introduction: The role of robotic lateral pelvic lymph node dissection (LPLND) for lateral pelvic nodal disease (LPND) in rectal cancer has yet to be investigated in the Western hemisphere. This study aims to investigate the safety and feasibility of robotic LPLND by utilising a well-established totally robotic TME protocol.
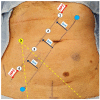
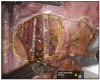
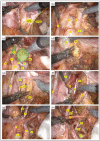
Methods: We conducted a retrospective study on 17 consecutive patients who underwent robotic LPLND for LPND ± TME for rectal cancer between 2015 and 2021. A single docking totally robotic approach from the left hip with full splenic mobilisation was performed using the X/Xi da Vinci platform. All patients underwent a tri-compartmental robotic en bloc excision of LPND with preservation of the obturator nerve and pelvic nerve plexus, leaving a well-skeletonised internal iliac vessel and its branches.
Results: The median operative time was 280 min, which was 40 min longer than our standard robotic TME. The median BMI was 26, and there were no conversions. The median inpatient stay was 7 days with no Clavien-Dindo > 3 complications. One patient (6%) developed local recurrence and metastatic disease within 5 months. The proportion of histologically confirmed LPND was 41%, of which 94% were well to moderately differentiated adenocarcinoma. Median pre-operative lateral pelvic node size was significantly higher in positive nodes (14 mm vs. 8 mm (p = 0.01)). All patients had clear resection margins on histology.
Discussion: Robotic LPLND is safe and feasible with good peri-operative and short-term outcomes, with the ergonomic advantages of a robotic TME docking protocol readily transferrable in LPLND.
Keywords: lateral pelvic lymph node dissection; minimally invasive surgery; robotic colorectal; robotic rectal cancer.
Conflict of interest statement
JSK is a proctor with intuitive surgical. All other authors declare no conflict of interest.
Figures
References
-
- Watanabe T., Muro K., Ajioka Y., Hashiguchi Y., Ito Y., Saito Y., Hamaguchi T., Ishida H., Ishiguro M., Ishihara S., et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2018;23:1–34. doi: 10.1007/s10147-017-1101-6. - DOI - PMC - PubMed
-
- Akiyoshi T., Watanabe T., Miyata S., Kotake K., Muto T., Sugihara K. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: Is it regional or distant disease? Ann. Surg. 2012;255:1129–1134. doi: 10.1097/SLA.0b013e3182565d9d. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

