The Role of IL-23 Inhibitors in Crohn's Disease
- PMID: 38202231
- PMCID: PMC10779938
- DOI: 10.3390/jcm13010224
The Role of IL-23 Inhibitors in Crohn's Disease
Abstract
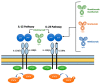
Promoting a Th17 pathogenic response, the interleukin (IL)-23 pathway is crucial in the pathophysiology of inflammatory bowel disease (IBD). With a favorable safety profile, ustekinumab, a monoclonal antibody targeting the shared p40 component of IL-12/23, is currently approved for the treatment of IBD in patients with disease refractory to corticosteroids and biologic drugs. Risankizumab, mirikizumab, and guselkumab are specific IL-23p19 antagonists tested for the treatment of Crohn's disease (CD). However, only risankizumab currently has been approved for its treatment. Trials with guselkumab and mirikizumab are currently ongoing, with promising preliminary efficacy and safety results. In this review, we provide a summary of the current knowledge about selective IL-23 inhibitors, focusing on their positioning in the therapeutic algorithm of patients with moderate to severe CD.
Keywords: Crohn’s disease; IL-23 inhibitors; brazikumab; guselkumab; inflammatory bowel disease; mirikizumab; risankizumab.
Conflict of interest statement
F D’Amico has served as a speaker for Sandoz, Janssen, Galapagos, and Omega Pharma; he also served as an advisory board member for Abbvie, Galapagos, and Nestlè. M Allocca has received consulting fees from Nikkiso Europe, Mundipharma, Janssen, AbbVie, Ferring, and Pfizer. F Furfaro received consulting fees from Amgen, AbbVie and lecture fees from Janssen and Pfizer. G Fiorino received consultancy fees from Ferring, MSD, AbbVie, Takeda, Janssen, Amgen, Sandoz, Samsung Bioepis, and Celltrion. Laurent Peyrin-Biroulet declares personal fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, OSE Immunotherapeutics, Enthera, Theravance, Pandion Therapeutics, Gossamer Bio, Viatris and Thermo Fisher, grants from Abbvie, MSD, Takeda and Fresenius Kabi, and stock options from CTMA. S Danese has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma and Vifor. J Fanizza, F Lusetti, E Fasulo, S Radice, A Zilli and TL Parigi declare no conflict of interest.
Figures
References
-
- Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources