Blood-Induced Arthropathy: A Major Disabling Complication of Haemophilia
- PMID: 38202232
- PMCID: PMC10779541
- DOI: 10.3390/jcm13010225
Blood-Induced Arthropathy: A Major Disabling Complication of Haemophilia
Abstract
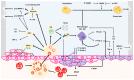
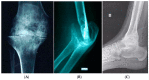
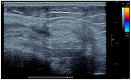
Haemophilic arthropathy (HA) is one of the most serious complications of haemophilia. It starts with joint bleeding, leading to synovitis which, in turn, can cause damage to the cartilage and subchondral bone, eventually inducing degenerative joint disease. Despite significant improvements in haemophilia treatment over the past two decades and recent guidelines from ISTH and WFH recommending FVIII trough levels of at least 3 IU/dL during prophylaxis, patients with haemophilia still develop joint disease. The pathophysiology of HA is complex, involving both inflammatory and degenerative components. Early diagnosis is key for proper management. Imaging can detect joint subclinical changes and influence prophylaxis. Magnetic resonance imagining (MRI) and ultrasound are the most frequently used methods in comprehensive haemophilia care centres. Biomarkers of joint health have been proposed to determine osteochondral joint deterioration, but none of these biomarkers has been validated or used in clinical practice. Early prophylaxis is key in all severe haemophilia patients to prevent arthropathy. Treatment is essentially based on prophylaxis intensification and chronic joint pain management. However, there remain significant gaps in the knowledge of the mechanisms responsible for HA and prognosis-influencing factors. Better understanding in this area could produce more effective interventions likely to ultimately prevent or attenuate the development of HA.
Keywords: MRI; arthropathy; haemophilia; joint bleed; prophylaxis; ultrasound.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Tissue-inhibitors of metalloproteinase-1 and vascular-endothelial growth-factor in severe haemophilia A children on low dose prophylactic recombinant factor VIII: Relation to subclinical arthropathy.Haemophilia. 2020 Jul;26(4):607-614. doi: 10.1111/hae.14041. Epub 2020 May 23. Haemophilia. 2020. PMID: 32445517
-
Haemophilia and joint disease: pathophysiology, evaluation, and management.J Comorb. 2011 Dec 27;1:51-59. doi: 10.15256/joc.2011.1.2. eCollection 2011. J Comorb. 2011. PMID: 29090136 Free PMC article. Review.
-
The hidden joint in children with haemophilia on prophylaxis.Thromb Res. 2023 Jun;226:86-92. doi: 10.1016/j.thromres.2023.04.012. Epub 2023 Apr 26. Thromb Res. 2023. PMID: 37130495
-
Prevention of joint damage in hemophilic children with early prophylaxis.Orthopade. 1999 Apr;28(4):341-346. doi: 10.1007/PL00003616. Orthopade. 1999. PMID: 28246905 English.
-
How to assess, detect, and manage joint involvement in the era of transformational therapies: Role of point-of-care ultrasound.Haemophilia. 2023 Jan;29(1):1-10. doi: 10.1111/hae.14657. Epub 2022 Sep 26. Haemophilia. 2023. PMID: 36163646 Review.
Cited by
-
A literature review of major surgery experience with emicizumab in people with hemophilia A without factor VIII inhibitors.Res Pract Thromb Haemost. 2025 Jan 31;9(1):102693. doi: 10.1016/j.rpth.2025.102693. eCollection 2025 Jan. Res Pract Thromb Haemost. 2025. PMID: 40093965 Free PMC article. Review.
-
Concizumab, a Non-Replacement Therapy for Persons with Hemophilia with Inhibitors.J Clin Med. 2025 Apr 25;14(9):2961. doi: 10.3390/jcm14092961. J Clin Med. 2025. PMID: 40363993 Free PMC article. Review.
-
Towards Personalized Treatment in Haemophilia: The Role of Genetic Factors in Iron and Heme Control to Identify Patients at Risk for Haemophilic Arthropathy.J Pers Med. 2024 Jan 28;14(2):145. doi: 10.3390/jpm14020145. J Pers Med. 2024. PMID: 38392579 Free PMC article.
References
-
- Brinkmann T., Kähnert H., Prohaska W., Nordfang O., Kleesiek K. Synthesis of tissue factor pathway inhibitor in human synovial cells and chondrocytes makes joints the predilected site of bleeding in haemophiliacs. Eur. J. Clin. Chem. Clin. Biochem. 1994;32:313–317. doi: 10.1515/cclm.1994.32.4.313. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

