The Clinical Analysis of Checkpoint Inhibitor Pneumonitis with Different Severities in Lung Cancer Patients: A Retrospective Study
- PMID: 38202262
- PMCID: PMC10779509
- DOI: 10.3390/jcm13010255
The Clinical Analysis of Checkpoint Inhibitor Pneumonitis with Different Severities in Lung Cancer Patients: A Retrospective Study
Abstract
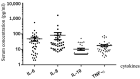
Immune-related adverse events (irAEs) of immunotherapy would lead to the temporary or permanent discontinuation of immune checkpoint inhibitors (ICIs). Among them, checkpoint inhibitor pneumonitis (CIP) is a potentially life-threatening irAE. This study aimed to identify the differences between patients with low-grade CIPs (grades 1-2) and high-grade CIPs (grades 3-5) and to explore the prognostic factors. We retrospectively reviewed the medical records of 916 lung cancer patients who were treated with ICIs. Patients with CIPs were identified after multidisciplinary discussion, and their clinical, laboratory, radiological, and follow-up data were analyzed. Among the 74 enrolled CIP patients, there were 31 low-grade CIPs and 43 high-grade CIPs. Compared with low-grade CIP patients, patients with high-grade CIPs were older (65.8 years vs. 61.5 years) and had lower serum albumin (35.2 g/L vs. 37.9 g/L), higher D-dimer (5.1 mg/L vs. 1.7 mg/L), and more pulmonary infectious diseases (32.6% vs. 6.5%) during follow-up. In addition, complication with pulmonary infectious diseases, management with intravenous immunoglobulin, tocilizumab, and longer duration of large dosage corticosteroids might be associated with worse outcomes for patients with CIPs. This study highlights potential risk factors for high-grade CIP and poor prognosis among lung cancer patients who were treated with anti-cancer ICIs.
Keywords: checkpoint inhibitor pneumonitis; immune-related adverse events; prognosis; severity grade.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Bronchoalveolar lavage fluid analysis in patients with checkpoint inhibitor pneumonitis.Cancer Immunol Immunother. 2024 Sep 13;73(11):235. doi: 10.1007/s00262-024-03834-y. Cancer Immunol Immunother. 2024. PMID: 39271538 Free PMC article.
-
[Clinical features and prognostic analysis of checkpoint inhibitor pneumonitis in patients with non-small cell lung cancer].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Mar 12;47(3):207-213. doi: 10.3760/cma.j.cn112147-20231003-00210. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38448169 Chinese.
-
Safety and efficacy of immunotherapy rechallenge following checkpoint inhibitor-related pneumonitis in advanced lung cancer patients: a retrospective multi-center cohort study.Transl Lung Cancer Res. 2022 Nov;11(11):2289-2305. doi: 10.21037/tlcr-22-732. Transl Lung Cancer Res. 2022. PMID: 36519018 Free PMC article.
-
Risk Factors for Immune Checkpoint Inhibitor-Related Pneumonitis in Cancer Patients: A Systemic Review and Meta-Analysis.Respiration. 2022;101(11):1035-1050. doi: 10.1159/000526141. Epub 2022 Sep 15. Respiration. 2022. PMID: 36108598
-
The association between the incidence risk of pneumonitis and PD-1/PD-L1 inhibitors in advanced NSCLC: A meta-analysis of randomized controlled trials.Int Immunopharmacol. 2021 Oct;99:108011. doi: 10.1016/j.intimp.2021.108011. Epub 2021 Aug 10. Int Immunopharmacol. 2021. PMID: 34426108
Cited by
-
[Research Progress of Anti-lung Cancer Drug-related Interstitial Lung Disease].Zhongguo Fei Ai Za Zhi. 2025 Apr 20;28(4):309-318. doi: 10.3779/j.issn.1009-3419.2025.106.11. Zhongguo Fei Ai Za Zhi. 2025. PMID: 40404479 Free PMC article. Review. Chinese.
-
Biomarkers and ImmuneScores in lung cancer: predictive insights for immunotherapy and combination treatment strategies.Biol Proced Online. 2025 Jul 10;27(1):25. doi: 10.1186/s12575-025-00287-0. Biol Proced Online. 2025. PMID: 40640703 Free PMC article. Review.
-
Identifying risk factors and biomarkers for severe CIP in lung cancer patients- a retrospective case series study.Immunotherapy. 2024;16(18-19):1131-1140. doi: 10.1080/1750743X.2024.2429369. Epub 2024 Nov 26. Immunotherapy. 2024. PMID: 39589860
References
-
- Liu T., Jin B., Chen J., Wang H., Lin S., Dang J., Li G. Comparative risk of serious and fatal treatment-related adverse events caused by 19 immune checkpoint inhibitors used in cancer treatment: A network meta-analysis. Ther. Adv. Med. Oncol. 2020;12:1758835920940927. doi: 10.1177/1758835920940927. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

