Imaging of Multiple Myeloma: Present and Future
- PMID: 38202271
- PMCID: PMC10780302
- DOI: 10.3390/jcm13010264
Imaging of Multiple Myeloma: Present and Future
Abstract
Multiple myeloma (MM) is the second most common adult hematologic malignancy, and early intervention increases survival in asymptomatic high-risk patients. Imaging is crucial for the diagnosis and follow-up of MM, as the detection of bone and bone marrow lesions often dictates the decision to start treatment. Low-dose whole-body computed tomography (CT) is the modality of choice for the initial assessment, and dual-energy CT is a developing technique with the potential for detecting non-lytic marrow infiltration and evaluating the response to treatment. Magnetic resonance imaging (MRI) is more sensitive and specific than 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) for the detection of small focal lesions and diffuse marrow infiltration. However, FDG-PET/CT is recommended as the modality of choice for follow-up. Recently, diffusion-weighted MRI has become a new technique for the quantitative assessment of disease burden and therapy response. Although not widespread, we address current proposals for structured reporting to promote standardization and diminish variations. This review provides an up-to-date overview of MM imaging, indications, advantages, limitations, and recommended reporting of each technique. We also cover the main differential diagnosis and pitfalls and discuss the ongoing controversies and future directions, such as PET-MRI and artificial intelligence.
Keywords: computed tomography; imaging; magnetic resonance imaging; multiple myeloma; positron emission tomography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

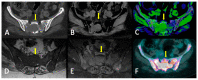

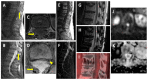
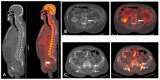
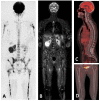

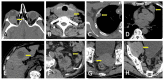
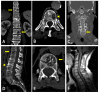
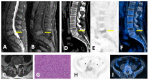
Similar articles
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Prospective Comparison of 18F-Choline Positron Emission Tomography/Computed Tomography (PET/CT) and 18F-Fluorodeoxyglucose (FDG) PET/CT in the Initial Workup of Multiple Myeloma: Study Protocol of a Prospective Imaging Trial.JMIR Res Protoc. 2020 Sep 10;9(9):e17850. doi: 10.2196/17850. JMIR Res Protoc. 2020. PMID: 32909953 Free PMC article.
-
A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma.Haematologica. 2007 Jan;92(1):50-5. doi: 10.3324/haematol.10554. Haematologica. 2007. PMID: 17229635
-
Modern imaging techniques for monitoring patients with multiple myeloma.Med Pharm Rep. 2022 Oct;95(4):377-384. doi: 10.15386/mpr-2215. Epub 2022 Oct 27. Med Pharm Rep. 2022. PMID: 36506611 Free PMC article. Review.
-
Skeletal Survey in Multiple Myeloma: Role of Imaging.Curr Med Imaging. 2021;17(8):956-965. doi: 10.2174/1573405617666210126155129. Curr Med Imaging. 2021. PMID: 33573573 Review.
Cited by
-
ESCRT may function as a tumor biomarker, transitioning from pan-cancer analysis to validation within breast cancer.Front Immunol. 2025 Mar 31;16:1531940. doi: 10.3389/fimmu.2025.1531940. eCollection 2025. Front Immunol. 2025. PMID: 40230849 Free PMC article.
-
Yearly Assessment of Bone Disease in Patients with Asymptomatic Multiple Myeloma Identifies Early Progression Events and Should Be the Standard Clinical Practice.J Clin Med. 2025 Mar 25;14(7):2224. doi: 10.3390/jcm14072224. J Clin Med. 2025. PMID: 40217676 Free PMC article.
-
CXCR4-targeted theranostics in acute leukemia: disrupting leukemic cell-microenvironment interactions with pentixafor and pentixather.Med Oncol. 2025 Aug 4;42(9):402. doi: 10.1007/s12032-025-02924-w. Med Oncol. 2025. PMID: 40754635 Review.
-
Enhancing prognosis in multiple myeloma bone disease: insights from a retrospective analysis of surgical interventions.Front Surg. 2024 Dec 19;11:1433265. doi: 10.3389/fsurg.2024.1433265. eCollection 2024. Front Surg. 2024. PMID: 39749125 Free PMC article.
-
Role of Imaging in Multiple Myeloma: A Potential Opportunity for Quantitative Imaging and Radiomics?Cancers (Basel). 2024 Dec 7;16(23):4099. doi: 10.3390/cancers16234099. Cancers (Basel). 2024. PMID: 39682285 Free PMC article. Review.
References
-
- Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.-V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed
-
- Dimopoulos M., Terpos E., Comenzo R.L., Tosi P., Beksac M., Sezer O., Siegel D., Lokhorst H., Kumar S., Rajkumar S.V., et al. International Myeloma Working Group Consensus Statement and Guidelines Regarding the Current Role of Imaging Techniques in the Diagnosis and Monitoring of Multiple Myeloma. Leukemia. 2009;23:1545–1556. doi: 10.1038/leu.2009.89. - DOI - PubMed
-
- Facon T., Dimopoulos M.A., Meuleman N., Belch A., Mohty M., Chen W.-M., Kim K., Zamagni E., Rodriguez-Otero P., Renwick W., et al. A Simplified Frailty Scale Predicts Outcomes in Transplant-Ineligible Patients with Newly Diagnosed Multiple Myeloma Treated in the FIRST (MM-020) Trial. Leukemia. 2020;34:224–233. doi: 10.1038/s41375-019-0539-0. - DOI - PMC - PubMed
-
- Palumbo A., Bringhen S., Mateos M.-V., Larocca A., Facon T., Kumar S.K., Offidani M., McCarthy P., Evangelista A., Lonial S., et al. Geriatric Assessment Predicts Survival and Toxicities in Elderly Myeloma Patients: An International Myeloma Working Group Report. Blood. 2015;125:2068–2074. doi: 10.1182/blood-2014-12-615187. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

