Safety and Tolerability of SGLT2 Inhibitors in Cardiac Amyloidosis-A Clinical Feasibility Study
- PMID: 38202290
- PMCID: PMC10780141
- DOI: 10.3390/jcm13010283
Safety and Tolerability of SGLT2 Inhibitors in Cardiac Amyloidosis-A Clinical Feasibility Study
Abstract
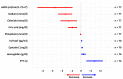
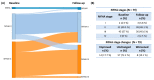
Sodium-glucose transport protein 2 inhibitors (SGLT2i) slow the progression of renal dysfunction and improve the prognosis of patients with heart failure. Amyloidosis constitutes an important subgroup for which evidence is lacking. Amyloidotic fibrils originating from misfolded transthyretin and light chains are the causal agents in ATTR and AL amyloidosis. In these most frequent subtypes, cardiac involvement is the most common organ manifestation. Because cardiac and renal function frequently deteriorate over time, even under best available treatment, SGLT2i emerge as a promising treatment option due to their reno- and cardioprotective properties. We retrospectively analyzed patients with cardiac amyloidosis, who received either dapagliflozin or empagliflozin. Out of 79 patients, 5.1% had urinary tract infections; 2 stopped SGLT2i therapy; and 2.5% died unrelated to the intake of SGLT2i. No genital mycotic infections were observed. As expected, a slight drop in the glomerular filtration rate was noted, while the NYHA functional status, cardiac and hepatic function, as well as the 6 min walk distance remained stable over time. These data provide a rationale for the use of SGLT2i in patients with amyloidosis and concomitant cardiac or renal dysfunction. Prospective randomized data are desired to confirm safety and to prove efficacy in this increasingly important group of patients.
Keywords: SGLT2 inhibitors; amyloidosis; chronic kidney disease; heart failure.
Conflict of interest statement
C.M. reports research cooperation with the University of Würzburg and Tomtec Imaging Systems funded by a research grant from the Bavarian Ministry of Economic Affairs, Regional Development and Energy, Germany; advisory and speakers honoraria as well as travel grants from Amgen, Tomtec, Alnylam, Sobi, Alexion, Janssen, Pfizer, Boehringer Ingelheim, AstraZeneca, Bayer, and EBR Systems. He was also principal investigator in trials sponsored by Alnylam, AstraZeneca, NovoNordisk, and Bayer. S.S. is supported by the CHFC Würzburg, and by the German Federal Ministry of Education and Research (BMBF). He has received consultancy and lecture fees as well as the reimbursement of travel costs from Alnylam, AstraZeneca, Bayer, Boehringer Ingelheim, IONIS, MSD, Novartis, Pfizer, and Servier. S.M. Ihne-Schubert received financial reimbursement for consulting, advisory board activities, speaker honoraria, and/or travel support to attend scientific meetings by Akcea Therapeutics, Alnylam, Pfizer, Janssen-Cilag, and Takeda, and further research funding from Pfizer and Akcea Therapeutics. Her internship was supported by ONLUS. She was a fellow of the local clinician scientist program of the IZKF Würzburg during the period 2018–2021. AP received financial reimbursement for consulting, advisory board activities, speaker honoraria, and/or travel support to attend scientific meetings by AKCEA, Alnylam, Pfizer, SOBI, AstraZeneca. She was also principal investigator in trial sponsored by Ionis and received research funding from Pfizer (ASPIRE 2019) and IZKF Würzburg, The other authors reported no conflicts of interest.
Figures
Similar articles
-
The Role of Sodium-Glucose Cotransporter-2 Inhibitors in Adults With Transthyretin Cardiac Amyloidosis: A Single-Center Retrospective Cohort Study.Cureus. 2024 Oct 30;16(10):e72725. doi: 10.7759/cureus.72725. eCollection 2024 Oct. Cureus. 2024. PMID: 39618657 Free PMC article.
-
Prognosis of Transthyretin Cardiac Amyloidosis Without Heart Failure Symptoms.JACC CardioOncol. 2022 Nov 15;4(4):442-454. doi: 10.1016/j.jaccao.2022.07.007. eCollection 2022 Nov. JACC CardioOncol. 2022. PMID: 36444226 Free PMC article.
-
Lessons from the first-in-human in vivo CRISPR/Cas9 editing of the TTR gene by NTLA-2001 trial in patients with transthyretin amyloidosis with cardiomyopathy.Glob Cardiol Sci Pract. 2023 Jan 30;2023(1):e202304. doi: 10.21542/gcsp.2023.4. eCollection 2023 Jan 30. Glob Cardiol Sci Pract. 2023. PMID: 37928601 Free PMC article.
-
The efficacy and safety of SGLT2 inhibitors in patients with non-diabetic chronic kidney disease: a systematic review and meta-analysis.Int Urol Nephrol. 2023 Dec;55(12):3167-3174. doi: 10.1007/s11255-023-03586-1. Epub 2023 Apr 13. Int Urol Nephrol. 2023. PMID: 37046125
-
Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus.Drugs. 2015 Jan;75(1):33-59. doi: 10.1007/s40265-014-0337-y. Drugs. 2015. PMID: 25488697 Review.
Cited by
-
Sodium-Glucose Cotransporter 2 Inhibitor Therapy in Different Scenarios of Heart Failure: An Overview of the Current Literature.Int J Mol Sci. 2024 Oct 25;25(21):11458. doi: 10.3390/ijms252111458. Int J Mol Sci. 2024. PMID: 39519011 Free PMC article. Review.
-
Predictors of developing renal dysfunction following diagnosis of transthyretin cardiac amyloidosis.Clin Cardiol. 2024 Jun;47(6):e24298. doi: 10.1002/clc.24298. Clin Cardiol. 2024. PMID: 38873847 Free PMC article.
-
Impact of SGLT2-Inhibitor Therapy on Survival in Patients with Transthyretin Amyloid Cardiomyopathy: Analysis of a Prospective Registry Study.J Clin Med. 2024 Oct 8;13(19):5966. doi: 10.3390/jcm13195966. J Clin Med. 2024. PMID: 39408026 Free PMC article.
-
Real-world characteristics and treatment of cardiac transthyretin amyloidosis: A multicentre, observational study.ESC Heart Fail. 2025 Apr;12(2):1203-1216. doi: 10.1002/ehf2.15126. Epub 2024 Nov 6. ESC Heart Fail. 2025. PMID: 39505353 Free PMC article.
-
Sodium-glucose cotransporter 2 inhibitors and outcomes in transthyretin amyloid cardiomyopathy: Systematic review and meta-analysis.Eur J Clin Invest. 2025 Jun;55(6):e14392. doi: 10.1111/eci.14392. Epub 2025 Jan 27. Eur J Clin Invest. 2025. PMID: 39868862 Free PMC article.
References
-
- Solomon S.D., McMurray J.J.V., Claggett B., de Boer R.A., DeMets D., Hernandez A.F., Inzucchi S.E., Kosiborod M.N., Lam C.S.P., Martinez F., et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. - DOI - PubMed
-
- McMurray J.J.V., DeMets D.L., Inzucchi S.E., Køber L., Kosiborod M.N., Langkilde A.M., Martinez F.A., Bengtsson O., Ponikowski P., Sabatine M.S., et al. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF) Eur. J. Heart Fail. 2019;21:665–675. doi: 10.1002/ejhf.1432. - DOI - PMC - PubMed
-
- Filippatos G., Butler J., Farmakis D., Zannad F., Ofstad A.P., Ferreira J.P., Green J.B., Rosenstock J., Schnaidt S., Brueckmann M., et al. Empagliflozin for Heart Failure with Preserved Left Ventricular Ejection Fraction With and Without Diabetes. Circulation. 2022;146:676–686. doi: 10.1161/CIRCULATIONAHA.122.059785. - DOI - PMC - PubMed
-
- Packer M., Anker S.D., Butler J., Filippatos G., Ferreira J.P., Pocock S.J., Carson P., Anand I., Doehner W., Haass M., et al. Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation. 2021;143:326–336. doi: 10.1161/CIRCULATIONAHA.120.051783. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

