Choice and Duration of Anticoagulation for Venous Thromboembolism
- PMID: 38202308
- PMCID: PMC10779515
- DOI: 10.3390/jcm13010301
Choice and Duration of Anticoagulation for Venous Thromboembolism
Abstract
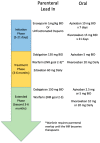
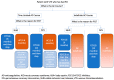
Venous thromboembolism (VTE) is a prevalent medical condition with high morbidity, mortality, and associated costs. Anticoagulation remains the main treatment for VTE, though the decision on when, how, and for how long to administer anticoagulants is increasingly complex. This review highlights the different phases of VTE management, with special circumstances for consideration such as antiphospholipid syndrome, coronary artery disease, cancer-associated thrombus, COVID-19, and future anticoagulation options. Anticoagulation management will continue to be a complex decision, applying evidence-based medicine to individual patients with the hope of maximizing effectiveness while minimizing risks.
Keywords: anticoagulation; cardio-vascular disease; deep vein thrombosis; pulmonary embolism; venous thromboembolism.
Conflict of interest statement
GDB: Grant Funding—Boston Scientific; Consulting—Pfizer, Bristol-Myers Squibb, Janssen, Bayer, AstraZeneca, Sanofi, Anthos, Abbott Vascular, Boston Scientific; DSMB—Translational Sciences (Clinical Events Adjudication Committee); Board of Directors—Anticoagulation Forum. AM and NH—none.
Figures
Similar articles
-
Treatment of DVT: how long is enough and how do you predict recurrence.J Thromb Thrombolysis. 2008 Feb;25(1):37-44. doi: 10.1007/s11239-007-0103-z. Epub 2007 Oct 1. J Thromb Thrombolysis. 2008. PMID: 17906973 Review.
-
Blood Being Tricky: Anticoagulation-Resistant Venous Thromboembolism (VTE).Cureus. 2022 Jun 13;14(6):e25914. doi: 10.7759/cureus.25914. eCollection 2022 Jun. Cureus. 2022. PMID: 35711251 Free PMC article.
-
Anticoagulation Treatment in Venous Thromboembolism: Options and Optimal Duration.Curr Pharm Des. 2022;28(4):296-305. doi: 10.2174/1381612827666211111150705. Curr Pharm Des. 2022. PMID: 34766887 Review.
-
Extended anticoagulation for venous thromboembolism: A survey of the American Venous Forum and the European Venous Forum.J Vasc Surg Venous Lymphat Disord. 2022 Sep;10(5):1012-1020.e3. doi: 10.1016/j.jvsv.2022.03.013. Epub 2022 May 11. J Vasc Surg Venous Lymphat Disord. 2022. PMID: 35561974
-
Which patients are at high risk of recurrent venous thromboembolism (deep vein thrombosis and pulmonary embolism)?Blood Adv. 2020 Nov 10;4(21):5595-5606. doi: 10.1182/bloodadvances.2020002268. Blood Adv. 2020. PMID: 33170937 Free PMC article. Review.
Cited by
-
Predictors of postoperative bleeding after minimally invasive bariatric surgery.Surg Endosc. 2024 Dec;38(12):7195-7201. doi: 10.1007/s00464-024-11284-x. Epub 2024 Oct 4. Surg Endosc. 2024. PMID: 39367135
-
The Role of Direct Oral Anticoagulants (DOACs) in Cancer-Associated Thrombosis: A Comprehensive Review of the Literature.Cureus. 2025 May 12;17(5):e83956. doi: 10.7759/cureus.83956. eCollection 2025 May. Cureus. 2025. PMID: 40510088 Free PMC article. Review.
-
Usefulness of Elastic Bandage Compression Compared to Calf Massage to Prevent Venous Thromboembolism-A Retrospective Evaluation.J Clin Med. 2024 Jul 25;13(15):4355. doi: 10.3390/jcm13154355. J Clin Med. 2024. PMID: 39124621 Free PMC article.
-
The Inflammatory Link of Rheumatoid Arthritis and Thrombosis: Pathogenic Molecular Circuits and Treatment Approaches.Curr Issues Mol Biol. 2025 Apr 18;47(4):291. doi: 10.3390/cimb47040291. Curr Issues Mol Biol. 2025. PMID: 40699690 Free PMC article. Review.
References
-
- CDC Data and Statistics on Venous Thromboembolism. [(accessed on 6 November 2023)]; Available online: https://www.cdc.gov/ncbddd/dvt/data.html.
-
- Stevens S.M., Woller S.C., Baumann Kreuziger L., Bounameaux H., Doerschug K., Geersing G.-J., Huisman M.V., Kearon C., King C.S., Knighton A.J., et al. Executive Summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:2247–2259. doi: 10.1016/j.chest.2021.07.056. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources