Enhanced Cytotoxicity and Antimelanoma Activity of Novel Semisynthetic Derivatives of Betulinic Acid with Indole Conjugation
- PMID: 38202344
- PMCID: PMC10780819
- DOI: 10.3390/plants13010036
Enhanced Cytotoxicity and Antimelanoma Activity of Novel Semisynthetic Derivatives of Betulinic Acid with Indole Conjugation
Abstract
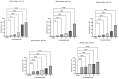
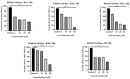
The prevalence and severity of skin cancer, specifically malignant melanoma, among Caucasians remains a significant concern. Natural compounds from plants have long been explored as potential anticancer agents. Betulinic acid (BI) has shown promise in its therapeutic properties, including its anticancer effects. However, its limited bioavailability has hindered its medicinal applications. To address this issue, two recently synthesized semisynthetic derivatives, N-(2,3-indolo-betulinoyl)diglycylglycine (BA1) and N-(2,3-indolo-betulinoyl)glycylglycine (BA2), were compared with previously reported compounds N-(2,3-indolo-betulinoyl)glycine (BA3), 2,3-indolo-betulinic acid (BA4), and BI. These compounds were evaluated for their effects on murine melanoma cells (B164A5) using various in vitro assays. The introduction of an indole framework at the C2 position of BI resulted in an increased cytotoxicity. Furthermore, the cytotoxicity of compound BA4 was enhanced by conjugating its carboxylic group with an amino acid residue. BA2 and BA3, with glycine and glycylglycine residues at C28, exhibited approximately 2.20-fold higher inhibitory activity compared to BA4. The safety assessment of the compounds on human keratinocytes (HaCaT) has revealed that concentrations up to 10 µM slightly reduced cell viability, while concentrations of 75 µM resulted in lower cell viability rates. LDH leakage assays confirmed cell membrane damage in B164A5 cells when exposed to the tested compounds. BA2 and BA3 exhibited the highest LDH release, indicating their strong cytotoxicity. The NR assay revealed dose-dependent lysosome disruption for BI and 2,3-indolo-betulinic acid derivatives, with BA1, BA2, and BA3 showing the most cytotoxic effects. Scratch assays demonstrated concentration-dependent inhibition of cell migration, with BA2 and BA3 being the most effective. Hoechst 3342 staining revealed that BA2 induced apoptosis, while BA3 induced necrosis at lower concentrations, confirming their anti-melanoma properties. In conclusion, the semisynthetic derivatives of BI, particularly BA2 and BA3, show promise as potential candidates for further research in developing effective anti-cancer therapies.
Keywords: 2,3-indolo-betulinic acid; B164A5 murine melanoma cells; glycine conjugates; melanoma.
Conflict of interest statement
Author Uldis Peipiņš was employed by the company Nature Science Technologies Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Comparison of In Vitro Antimelanoma and Antimicrobial Activity of 2,3-Indolo-betulinic Acid and Its Glycine Conjugates.Plants (Basel). 2023 Mar 9;12(6):1253. doi: 10.3390/plants12061253. Plants (Basel). 2023. PMID: 36986941 Free PMC article.
-
Apoptotic activity of betulinic acid derivatives on murine melanoma B16 cell line.Eur J Pharmacol. 2004 Sep 13;498(1-3):71-8. doi: 10.1016/j.ejphar.2004.07.103. Eur J Pharmacol. 2004. PMID: 15363977
-
The new esters derivatives of betulin and betulinic acid in epidermoid squamous carcinoma treatment - In vitro studies.Biomed Pharmacother. 2015 May;72:91-7. doi: 10.1016/j.biopha.2015.04.003. Epub 2015 Apr 13. Biomed Pharmacother. 2015. PMID: 26054680
-
Betulinic acid derivatives as anticancer agents: structure activity relationship.Anticancer Agents Med Chem. 2006 May;6(3):271-9. doi: 10.2174/187152006776930846. Anticancer Agents Med Chem. 2006. PMID: 16712455 Review.
-
Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection.Med Res Rev. 2004 Jan;24(1):90-114. doi: 10.1002/med.10053. Med Res Rev. 2004. PMID: 14595673 Review.
Cited by
-
Fe3O4@β-cyclodextrin Nanosystem: A Promising Adjuvant Approach in Cancer Treatment.Nanomaterials (Basel). 2025 Aug 4;15(15):1192. doi: 10.3390/nano15151192. Nanomaterials (Basel). 2025. PMID: 40801730 Free PMC article.
-
Cutaneous Evaluation of Fe3O4 Nanoparticles: An Assessment Based on 2D and 3D Human Epidermis Models Under Standard and UV Conditions.Int J Nanomedicine. 2025 Mar 20;20:3653-3670. doi: 10.2147/IJN.S513423. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40130196 Free PMC article.
-
Chemopreventive and Anticancer Activity of Selected Triterpenoids in Melanoma.Cancers (Basel). 2025 May 11;17(10):1625. doi: 10.3390/cancers17101625. Cancers (Basel). 2025. PMID: 40427124 Free PMC article. Review.
References
-
- Siddiqui A.J., Jahan S., Singh R., Saxena J., Ashraf S.A., Khan A., Choudhary R.K., Balakrishnan S., Badraoui R., Bardakci F., et al. Review Article Plants in Anticancer Drug Discovery: From Molecular Mechanism to Chemoprevention. BioMed Res. Int. 2022;2022:5425485. doi: 10.1155/2022/5425485. - DOI - PMC - PubMed
-
- Cragg G.M., Newman D.J., Snader K.M. The Influence of Natural Products upon Drug Discovery. Nat. Prod. Rep. 2000;17:215–234. - PubMed
-
- Bhusnure O.G., Shinde M.C., Vijayendra S.S.M., Gholve S.B., Giram P.S., Birajdar M.J. Phytopharmaceuticals: An Emerging Platform for Innovation and Development of New Drugs from Botanicals. J. Drug Deliv. Ther. 2019;9:1046–1057.
-
- Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

