Influences of Growth Stage and Ensiling Time on Fermentation Characteristics, Nitrite, and Bacterial Communities during Ensiling of Alfalfa
- PMID: 38202392
- PMCID: PMC10780930
- DOI: 10.3390/plants13010084
Influences of Growth Stage and Ensiling Time on Fermentation Characteristics, Nitrite, and Bacterial Communities during Ensiling of Alfalfa
Abstract
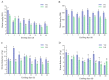
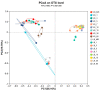
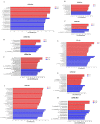
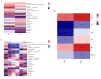
This study examined the impacts of growth stage and ensiling duration on the fermentation characteristics, nitrite content, and bacterial communities during the ensiling of alfalfa. Harvested alfalfa was divided into two groups: vegetative growth stage (VG) and late budding stage (LB). The fresh alfalfa underwent wilting until reaching approximately 65% moisture content, followed by natural fermentation. The experiment followed a completely randomized design, with samples collected after the wilting of alfalfa raw materials (MR) and on days 1, 3, 5, 7, 15, 30, and 60 of fermentation. The growth stage significantly influenced the chemical composition of alfalfa, with crude protein content being significantly higher in the vegetative growth stage alfalfa compared to that in the late budding stage (p < 0.05). Soluble carbohydrates, neutral detergent fiber, and acid detergent fiber content were significantly lower in the vegetative growth stage compared to the late budding stage (p < 0.05). Nitrite content, nitrate content, nitrite reductase activity, and nitrate reductase activity were all significantly higher in the vegetative growth stage compared to the late budding stage (p < 0.05). In terms of fermentation parameters, silage from the late budding stage exhibited superior characteristics compared to that from the vegetative growth stage. Compared to the alfalfa silage during the vegetative growth stage, the late budding stage group exhibited a higher lactate content and lower pH level. Notably, butyric acid was only detected in the silage from the vegetative growth stage group. Throughout the ensiling process, nitrite content, nitrate levels, nitrite reductase activity, and nitrate reductase activity decreased in both treatment groups. The dominant lactic acid bacteria differed between the two groups, with Enterococcus being predominant in vegetative growth stage alfalfa silage, and Weissella being predominant in late budding stage silage, transitioning to Lactiplantibacillus in the later stages of fermentation. On the 3rd day of silage fermentation, the vegetative growth stage group exhibited the highest abundance of Enterococcus, which subsequently decreased to its lowest level on the 15th day. Correlation analysis revealed that lactic acid bacteria, including Limosilactobacillus, Levilactobacillus, Loigolactobacillus, Pediococcus, Lactiplantibacillus, and Weissella, played a key role in nitrite and nitrate degradation in alfalfa silage. The presence of nitrite may be linked to Erwinia, unclassified_o__Enterobacterales, Pantoea, Exiguobacterium, Enterobacter, and Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium.
Keywords: alfalfa silage; bacterial communities; enzyme activity; growth stage; nitrite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Bacterial Community and Fermentation Quality of Ensiling Alfalfa With Commercial Lactic Acid Bacterial Additives.Front Microbiol. 2022 Apr 22;13:836899. doi: 10.3389/fmicb.2022.836899. eCollection 2022. Front Microbiol. 2022. PMID: 35531295 Free PMC article.
-
Dynamics of microbial community during ensiling direct-cut alfalfa with and without LAB inoculant and sugar.J Appl Microbiol. 2017 Jun;122(6):1456-1470. doi: 10.1111/jam.13456. Epub 2017 May 3. J Appl Microbiol. 2017. PMID: 28370869
-
Varieties and ensiling: Impact on chemical composition, fermentation quality and bacterial community of alfalfa.Front Microbiol. 2023 Jan 11;13:1091491. doi: 10.3389/fmicb.2022.1091491. eCollection 2022. Front Microbiol. 2023. PMID: 36713170 Free PMC article.
-
Natural fermentation quality, bacteria, and functional profiles of three cuttings of alfalfa silage in a year in Inner Mongolia, China.Front Microbiol. 2023 Mar 9;14:1083620. doi: 10.3389/fmicb.2023.1083620. eCollection 2023. Front Microbiol. 2023. PMID: 36970661 Free PMC article.
-
The potential and effects of saline-alkali alfalfa microbiota under salt stress on the fermentation quality and microbial.BMC Microbiol. 2021 May 19;21(1):149. doi: 10.1186/s12866-021-02213-2. BMC Microbiol. 2021. PMID: 34011262 Free PMC article.
Cited by
-
Analysis of full length transcriptome and resistance characteristics of Atraphaxis bracteata under drought.Sci Rep. 2025 Jan 4;15(1):807. doi: 10.1038/s41598-024-80831-2. Sci Rep. 2025. PMID: 39755718 Free PMC article.
References
-
- Bagavathiannan M.V., Gulden R.H., Begg G.S., Acker R.C.V. The demography of feral alfalfa (Medicago sativa L.) populations occurring in roadside habitats in Southern Manitoba, Canada: Implications for novel trait confinement. Environ. Sci. Pollut. Res. Int. 2010;17:1448–1459. doi: 10.1007/s11356-010-0330-2. - DOI - PubMed
-
- Mielmann A. The utilisation of lucerne (Medicago sativa): A review. Br. Food J. 2013;115:590–600. doi: 10.1108/00070701311317865. - DOI
-
- Rotz C.A., Abrams S., Davis R. Alfalfa Drying, Loss and Quality as Influenced by Mechanical and Chemical Conditioning. Trans. ASAE. 1987;30:0630–0635. doi: 10.13031/2013.30451. - DOI
-
- Hlödversson R., Kaspersson A. Nutrient losses during deterioration of hay in relation to changes in biochemical composition and microbial growth. Anim. Feed Sci. Technol. 1986;15:149–165. doi: 10.1016/0377-8401(86)90022-2. - DOI
LinkOut - more resources
Full Text Sources

