Elicitation Induced α-Amyrin Synthesis in Tylophora indica In Vitro Cultures and Comparative Phytochemical Analyses of In Vivo and Micropropagated Plants
- PMID: 38202430
- PMCID: PMC10780849
- DOI: 10.3390/plants13010122
Elicitation Induced α-Amyrin Synthesis in Tylophora indica In Vitro Cultures and Comparative Phytochemical Analyses of In Vivo and Micropropagated Plants
Abstract
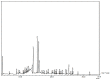
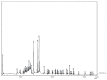
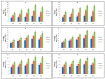
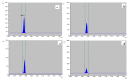
Tylophora indica (Burm. f.) Merrill is an endangered medicinal plant that possesses various active agents, such as tylophorinine, kaempferol, quercetin, α-amyrin and beta-sitosterol, with multiple medicinal benefits. α-amyrin, a triterpenoid, is widely known for its antimicrobial, anti-inflammatory, gastroprotective and hepatoprotective properties. In this study, we investigated the metabolite profiling of tissues and the effects of cadmium chloride and chitosan on in vitro accumulation of alkaloids in T. indica. First, the callus was induced from the leaf in 2,4-D-, NAA- and/or BAP-fortified MS medium. Subsequent shoot formation through organogenesis and in vitro roots was later induced. Gas chromatography-mass spectrometry (GC-MS)-based phytochemical profiling of methanolic extracts of in vivo and in vitro regenerated plants was conducted, revealing the presence of the important phytocompounds α-amyrin, lupeol, beta-sitosterol, septicine, tocopherol and several others. Different in vitro grown tissues, like callus, leaf and root, were elicited with cadmium chloride (0.1-0.4 mg L-1) and chitosan (1-50 mg L-1) to evaluate the effect of elicitation on α-amyrin accumulation, measured with high-performance thin layer chromatography (HPTLC). CdCl2 and chitosan showed improved sugar (17.24 and 15.04 mg g-1 FW, respectively), protein (10.76 and 9.99 mg g-1 FW, respectively) and proline (7.46 and 7.12 mg g-1 FW), especially at T3 (0.3 and 25 mg L-1), in the leaf as compared to those of the control and other tissues. The antioxidant enzyme activities were also evaluated under an elicitated stress situation, wherein catalase (CAT), superoxide dismutase (SOD) and ascorbate peroxidase (APX) displayed the highest activities in the leaf at T4 of both of the two elicitors. The α-amyrin yield was quantified with HPTLC in all tested tissues (leaf, callus and root) and had an Rf = 0.62 at 510 nm wavelength. Among all the concentrations tested, the T3 treatment (0.3 mg L-1 of cadmium chloride and 25 mg L-1 of chitosan) had the best influence on accumulation, irrespective of the tissues, with the maximum being in the leaf (2.72 and 2.64 μg g-1 DW, respectively), followed by the callus and root. Therefore, these results suggest future opportunities of elicitors in scaling up the production of important secondary metabolites to meet the requirements of the pharmaceutical industry.
Keywords: GC–MS analysis; HPTLC; Tylophora indica; biochemical analyses; elicitors; in vitro culture; α-amyrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Integrated GC-MS and UPLC-ESI-QTOF-MS based untargeted metabolomics analysis of in vitro raised tissues of Digitalis purpurea L.Front Plant Sci. 2024 Aug 22;15:1433634. doi: 10.3389/fpls.2024.1433634. eCollection 2024. Front Plant Sci. 2024. PMID: 39239200 Free PMC article.
-
Cadmium chloride (CdCl2) elicitation improves reserpine and ajmalicine yield in Rauvolfia serpentina as revealed by high-performance thin-layer chromatography (HPTLC).3 Biotech. 2020 Aug;10(8):344. doi: 10.1007/s13205-020-02339-6. Epub 2020 Jul 20. 3 Biotech. 2020. PMID: 32714739 Free PMC article.
-
Genome size and gas chromatography-mass spectrometry (GC-MS) analysis of field-grown and in vitro regenerated Pluchea lanceolata plants.J Appl Genet. 2023 Feb;64(1):1-21. doi: 10.1007/s13353-022-00727-7. Epub 2022 Sep 30. J Appl Genet. 2023. PMID: 36175751 Free PMC article.
-
Tylophora indica (Burm. f.) merr: An insight into phytochemistry and pharmacology.J Ethnopharmacol. 2020 Nov 15;262:113122. doi: 10.1016/j.jep.2020.113122. Epub 2020 Jul 27. J Ethnopharmacol. 2020. PMID: 32730871 Review.
-
Micropropagation and genetic transformation of Tylophora indica (Burm. f.) Merr.: a review.Plant Cell Rep. 2016 Nov;35(11):2207-2225. doi: 10.1007/s00299-016-2041-8. Epub 2016 Aug 23. Plant Cell Rep. 2016. PMID: 27553812 Review.
Cited by
-
Association Between Gall Structural and Metabolic Complexity: Evidence from Pistacia palaestina.Plants (Basel). 2025 Feb 26;14(5):721. doi: 10.3390/plants14050721. Plants (Basel). 2025. PMID: 40094633 Free PMC article.
-
Integrated GC-MS and UPLC-ESI-QTOF-MS based untargeted metabolomics analysis of in vitro raised tissues of Digitalis purpurea L.Front Plant Sci. 2024 Aug 22;15:1433634. doi: 10.3389/fpls.2024.1433634. eCollection 2024. Front Plant Sci. 2024. PMID: 39239200 Free PMC article.
-
Phytocompounds and Regulation of Flavonoids in In Vitro-Grown Safflower Plant Tissue by Abiotic Elicitor CdCl2.Metabolites. 2024 Feb 16;14(2):127. doi: 10.3390/metabo14020127. Metabolites. 2024. PMID: 38393019 Free PMC article.
References
-
- Akram W., Saeed T., Ahmad A., Yasin N.A., Akbar M., Khan W.U., Ahmed S., Guo J., Luo W., Wu T., et al. Liquiritin Elicitation Can Increase the Content of Medicinally Important Glucosinolates and Phenolic Compounds in Chinese Kale Plants. J. Sci. Food Agric. 2020;100:1616–1624. doi: 10.1002/jsfa.10170. - DOI - PubMed
-
- Golkar P., Taghizadeh M., Yousefian Z. The Effects of Chitosan and Salicylic Acid on Elicitation of Secondary Metabolites and Antioxidant Activity of Safflower under in Vitro Salinity Stress. Plant Cell Tissue Organ. Cult. 2019;137:575–585. doi: 10.1007/s11240-019-01592-9. - DOI
-
- Dey A., Nandy S., Nongdam P., Tikendra L., Mukherjee A., Mukherjee S., Pandey D.K. Methyl Jasmonate and Salicylic Acid Elicit Indole Alkaloid Production and Modulate Antioxidant Defence and Biocidal Properties in Rauvolfia serpentina Benth. ex Kurz. in Vitro Cultures. S. Afr. J. Bot. 2020;135:1–17. doi: 10.1016/j.sajb.2020.07.020. - DOI
-
- Ayoola-Oresanya I.O., Sonibare M.A., Gueye B., Abberton M.T., Morlock G.E. Elicitation of Antioxidant Metabolites in Musa Species in Vitro Shoot Culture Using Sucrose, Temperature and Jasmonic Acid. Plant Cell Tissue Organ. Cult. 2021;146:225–236. doi: 10.1007/s11240-021-02062-x. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

