Recent Progress on Functionalized Graphene Quantum Dots and Their Nanocomposites for Enhanced Gas Sensing Applications
- PMID: 38202466
- PMCID: PMC10780593
- DOI: 10.3390/nano14010011
Recent Progress on Functionalized Graphene Quantum Dots and Their Nanocomposites for Enhanced Gas Sensing Applications
Abstract
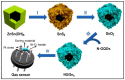
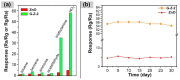
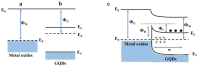
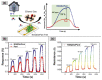
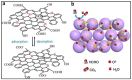
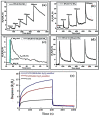
Gas-sensing technology has witnessed significant advancements that have been driven by the emergence of graphene quantum dots (GQDs) and their tailored nanocomposites. This comprehensive review surveys the recent progress made in the construction methods and applications of functionalized GQDs and GQD-based nanocomposites for gas sensing. The gas-sensing mechanisms, based on the Fermi-level control and charge carrier depletion layer theory, are briefly explained through the formation of heterojunctions and the adsorption/desorption principle. Furthermore, this review explores the enhancements achieved through the incorporation of GQDs into nanocomposites with diverse matrices, including polymers, metal oxides, and 2D materials. We also provide an overview of the key progress in various hazardous gas sensing applications using functionalized GQDs and GQD-based nanocomposites, focusing on key detection parameters such as sensitivity, selectivity, stability, response and recovery time, repeatability, and limit of detection (LOD). According to the most recent data, the normally reported values for the LOD of various toxic gases using GQD-based sensors are in the range of 1-10 ppm. Remarkably, some GQD-based sensors exhibit extremely low detection limits, such as N-GQDs/SnO2 (0.01 ppb for formaldehyde) and GQD@SnO2 (0.10 ppb for NO2). This review provides an up-to-date perspective on the evolving landscape of functionalized GQDs and their nanocomposites as pivotal components in the development of advanced gas sensors.
Keywords: GQDs based nanocomposite; functionalized GQDs; gas sensing mechanism; improved sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Role of graphene quantum dots with discrete band gaps on SnO2 nanodomes for NO2 gas sensors with an ultralow detection limit.Nanoscale Adv. 2023 Apr 28;5(10):2767-2775. doi: 10.1039/d2na00925k. eCollection 2023 May 16. Nanoscale Adv. 2023. PMID: 37205284 Free PMC article.
-
Recent Advances of Graphene Quantum Dots in Chemiresistive Gas Sensors.Nanomaterials (Basel). 2023 Oct 30;13(21):2880. doi: 10.3390/nano13212880. Nanomaterials (Basel). 2023. PMID: 37947725 Free PMC article. Review.
-
Three-Dimensional MoS2/Reduced Graphene Oxide Nanosheets/Graphene Quantum Dots Hybrids for High-Performance Room-Temperature NO2 Gas Sensors.Nanomaterials (Basel). 2022 Mar 9;12(6):901. doi: 10.3390/nano12060901. Nanomaterials (Basel). 2022. PMID: 35335714 Free PMC article.
-
N-Doped Graphene Quantum Dot-Decorated Three-Dimensional Ordered Macroporous In2O3 for NO2 Sensing at Low Temperatures.ACS Appl Mater Interfaces. 2020 Jul 29;12(30):34245-34253. doi: 10.1021/acsami.0c03369. Epub 2020 Jul 20. ACS Appl Mater Interfaces. 2020. PMID: 32633129
-
Recent progress on graphene quantum dots-based fluorescence sensors for food safety and quality assessment applications.Compr Rev Food Sci Food Saf. 2021 Nov;20(6):5765-5801. doi: 10.1111/1541-4337.12834. Epub 2021 Oct 2. Compr Rev Food Sci Food Saf. 2021. PMID: 34601802 Review.
References
-
- Daglar H., Altintas C., Erucar I., Heidari G., Zare E.N., Moradi O., Srivastava V., Iftekhar S., Keskin S., Sillanpää M. Metal-organic framework-based materials for the abatement of air pollution and decontamination of wastewater. Chemosphere. 2022;303:135082. doi: 10.1016/j.chemosphere.2022.135082. - DOI - PubMed
-
- Goel N., Kunal K., Kushwaha A., Kumar M. Metal oxide semiconductors for gas sensing. Eng. Rep. 2023;5:e12604. doi: 10.1002/eng2.12604. - DOI
-
- Souri M., Amoli H.S. Gas sensing mechanisms in ABO3 perovskite materials at room temperature: A review. Mater. Sci. Semicond. Process. 2023;156:107271. doi: 10.1016/j.mssp.2022.107271. - DOI
Publication types
LinkOut - more resources
Full Text Sources

