Terpenes-Modified Lipid Nanosystems for Temozolomide, Improving Cytotoxicity against Glioblastoma Human Cancer Cells In Vitro
- PMID: 38202510
- PMCID: PMC10780480
- DOI: 10.3390/nano14010055
Terpenes-Modified Lipid Nanosystems for Temozolomide, Improving Cytotoxicity against Glioblastoma Human Cancer Cells In Vitro
Abstract
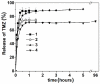
Currently, increasing the efficiency of glioblastoma treatment is still an unsolved problem. In this study, a combination of promising approaches was proposed: (i) an application of nanotechnology approach to create a new terpene-modified lipid system (7% w/w), using soybean L-α-phosphatidylcholine, N-carbonyl-methoxypolyethylene glycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine for delivery of the chemotherapy drug, temozolomide (TMZ, 1 mg/mL); (ii) use of TMZ associated with natural compounds-terpenes (1% w/w) abietic acid and Abies sibirica Ledeb. resin (A. sibirica). Different concentrations and combinations of terpene-lipid systems were employed to treat human cancer cell lines T 98G (glioblastoma), M-Hela (carcinoma of the cervix) and human liver cell lines (Chang liver). The terpene-lipid systems appeared to be unilamellar and of spherical shape under transmission electron microscopy (TEM). The creation of a TMZ-loaded terpene-lipid nanosystem was about 100 nm in diameter with a negative surface charge found by dynamic light scattering. The 74% encapsulation efficiency allowed the release time of TMZ to be prolonged. The modification by terpenes of TMZ-loaded lipid nanoparticles improved by four times the cytotoxicity against human cancer T 98G cells and decreased the cytotoxicity against human normal liver cells. Terpene-modified delivery lipid systems are of potential interest as a combination therapy.
Keywords: Abies sibirica Ledeb. resin; abietic acid; lipid nanoparticles; temozolomide; terpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Temozolomide-loaded PLGA nanoparticles to treat glioblastoma cells: a biophysical and cell culture evaluation.Neurol Res. 2016 Jan;38(1):51-9. doi: 10.1080/01616412.2015.1133025. Epub 2016 Feb 22. Neurol Res. 2016. PMID: 26905383
-
Oncolytic Newcastle Disease Virus Co-Delivered with Modified PLGA Nanoparticles Encapsulating Temozolomide against Glioblastoma Cells: Developing an Effective Treatment Strategy.Molecules. 2022 Sep 6;27(18):5757. doi: 10.3390/molecules27185757. Molecules. 2022. PMID: 36144488 Free PMC article.
-
Tri-block copolymer nanoparticles modified with folic acid for temozolomide delivery in glioblastoma.Int J Biochem Cell Biol. 2019 Mar;108:72-83. doi: 10.1016/j.biocel.2019.01.010. Epub 2019 Jan 17. Int J Biochem Cell Biol. 2019. PMID: 30660689
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
Current advances in temozolomide encapsulation for the enhancement of glioblastoma treatment.Theranostics. 2023 May 8;13(9):2734-2756. doi: 10.7150/thno.82005. eCollection 2023. Theranostics. 2023. PMID: 37284445 Free PMC article. Review.
Cited by
-
Lavender Essential Oil and Its Terpenic Components Negatively Affect Tumor Properties in a Cell Model of Glioblastoma.Molecules. 2024 Dec 22;29(24):6044. doi: 10.3390/molecules29246044. Molecules. 2024. PMID: 39770132 Free PMC article.
-
Intranasal Terpene Treatment for Glioblastoma: the Neuro-Oncological Potential of Perillyl Alcohol.Neurochem Res. 2025 Jul 29;50(4):254. doi: 10.1007/s11064-025-04505-9. Neurochem Res. 2025. PMID: 40728696 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

