A Nanoporous Polymer Modified with Hexafluoroisopropanol to Detect Dimethyl Methylphosphonate
- PMID: 38202543
- PMCID: PMC10781009
- DOI: 10.3390/nano14010089
A Nanoporous Polymer Modified with Hexafluoroisopropanol to Detect Dimethyl Methylphosphonate
Abstract
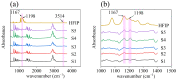
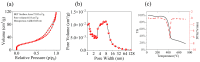
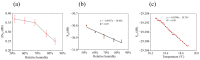
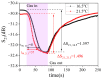
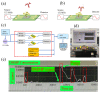
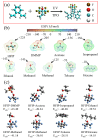
The increasing threat of nerve agents has prompted the need for gas sensors with fast response, high sensitivity, and good stability. In this work, the hexafluoroisopropanol functional group was modified on a porous aromatic framework material, which served as a sensitive material for detecting dimethyl methylphosphonate. A nerve agent sensor was made by coating sensitive materials on a surface acoustic wave device. Lots of pores in sensitive materials effectively increase the specific surface area and provide channels for diffusion of gas molecules. The introduction of hexafluoroisopropanols enables the sensor to specifically adsorb dimethyl methylphosphonate and improves the selectivity of the sensor. As a result, the developed gas sensor was able to detect dimethyl methylphosphonate at 0.8 ppm with response/recovery times of 29.8/43.8 s, and the detection limit of the gas sensor is about 0.11 ppm. The effects of temperature and humidity on the sensor were studied. The results show that the baseline of the sensor has a linear relationship with temperature and humidity, and the temperature and humidity have a significant effect on the response of the sensor. Furthermore, a device for real-time detection of nerve agent is reported. This work provides a new strategy for developing a gas sensor for detecting nerve agents.
Keywords: dimethyl methylphosphonate; gas sensor; hexafluoroisopropanol; surface acoustic wave.
Conflict of interest statement
The authors declare that they have no competing financial interest or personal relationships that could influence the work reported in this study.
Figures






Similar articles
-
High-performance p-hexafluoroisopropanol phenyl functionalized multi-walled carbon nanotube film on surface acoustic wave device for organophosphorus vapor detection.Nanotechnology. 2022 Jun 20;33(37). doi: 10.1088/1361-6528/ac7242. Nanotechnology. 2022. PMID: 35605577
-
p-Hexafluoroisopropanol phenyl functionalized graphene for QCM based detection of dimethyl methylphosphonate, a simulant of the nerve agent sarin.RSC Adv. 2018 Feb 21;8(15):8240-8245. doi: 10.1039/c7ra12272a. eCollection 2018 Feb 19. RSC Adv. 2018. PMID: 35541990 Free PMC article.
-
Surface acoustic wave platform integrated with ultraviolet activated rGO-SnS2 nanocomposites to achieve ppb-level dimethyl methylphosphonate detection at room-temperature.Talanta. 2025 Jan 1;282:127063. doi: 10.1016/j.talanta.2024.127063. Epub 2024 Oct 17. Talanta. 2025. PMID: 39423635
-
Enhanced dimethyl methylphosphonate detection based on two-dimensional WSe2 nanosheets at room temperature.Analyst. 2021 Jan 7;145(24):8059-8067. doi: 10.1039/d0an01671c. Analyst. 2021. PMID: 33078181
-
Sensitive Materials Used in Surface Acoustic Wave Gas Sensors for Detecting Sulfur-Containing Compounds.Polymers (Basel). 2024 Feb 6;16(4):457. doi: 10.3390/polym16040457. Polymers (Basel). 2024. PMID: 38399835 Free PMC article. Review.
References
-
- Lai Y.-T., Kuo J.-C., Yang Y.-J. Polymer-dispersed liquid crystal doped with carbon nanotubes for dimethyl methylphosphonate vapor-sensing application. Appl. Phys. Lett. 2013;102:191912. doi: 10.1063/1.4804297. - DOI
-
- Pagidi S., Pasupuleti K.S., Reddeppa M., Ahn S., Kim Y., Kim J.-H., Kim M.-D., Lee S.H., Jeon M.Y. Resistive type NO2 gas sensing in polymer-dispersed liquid crystals with functionalized-carbon nanotubes dopant at room temperature. Sens. Actuators B Chem. 2022;370:132482. doi: 10.1016/j.snb.2022.132482. - DOI
-
- Yang Z., Zhao L., Zhang Y., Xing Y., Fei T., Liu S., Zhang T. DMMP sensors based on Au-SnO2 hybrids prepared through colloidal assembly approach: Gas sensing performances and mechanism study. Sens. Actuators B Chem. 2022;369:132278. doi: 10.1016/j.snb.2022.132278. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

