In Vivo Evaluation of Bone Regenerative Capacity of the Novel Nanobiomaterial: β-Tricalcium Phosphate Polylactic Acid-co-Glycolide (β-TCP/PLLA/PGA) for Use in Maxillofacial Bone Defects
- PMID: 38202548
- PMCID: PMC10780666
- DOI: 10.3390/nano14010091
In Vivo Evaluation of Bone Regenerative Capacity of the Novel Nanobiomaterial: β-Tricalcium Phosphate Polylactic Acid-co-Glycolide (β-TCP/PLLA/PGA) for Use in Maxillofacial Bone Defects
Abstract
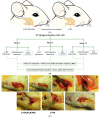
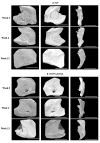
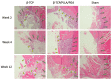
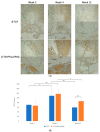
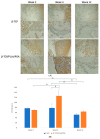
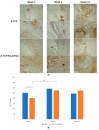
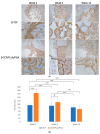
Maxillofacial bone defects are treated by autografting or filling with synthetic materials in various forms and shapes. Electrospun nanobiomaterials are becoming popular due to their easy placement and handling; combining ideal biomaterials extrapolates better outcomes. We used a novel electrospun cotton-like fiber made from two time-tested bioresorbable materials, β-TCP and PLLA/PGA, to check the feasibility of its application to maxillofacial bone defects through an in vivo rat mandibular bone defect model. Novel β-TCP/PLLA/PGA and pure β-TCP blocks were evaluated for new bone regeneration through assessment of bone volume, inner defect diameter reduction, and bone mineral density. Bioactive/osteoconductivity was checked by scoring the levels of Runt-related transcription factor x, Leptin Receptor, Osteocalcin, and Periostin biomarkers. Bone regeneration in both β-TCP/PLLA/PGA and β-TCP was comparable at initial timepoints. Osteogenic cell accumulation was greater in β-TCP/PLLA/PGA than in β-TCP at initial as well as late phases. Periostin expression was more marked in β-TCP/PLLA/PGA. This study demonstrated comparable results between β-TCP/PLLA/PGA and β-TCP in terms of bone regeneration and bioactivity, even with a small material volume of β-TCP/PLLA/PGA and a decreased percentage of β-TCP. Electrospun β-TCP/PLLA/PGA is an ideal nanobiomaterial for inducing bone regeneration through osteoconductivity and bioresorbability in bony defects of the maxillofacial region.
Keywords: electrospun nanobiomaterial; maxillofacial bone regeneration; synthetic/artificial bone graft substitutes; β-TCP/PLLA/PGA cotton-like fiber; β-tricalcium phosphate.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Bone Regeneration Capacity of Newly Developed Uncalcined/Unsintered Hydroxyapatite and Poly-l-lactide-co-glycolide Sheet in Maxillofacial Surgery: An In Vivo Study.Nanomaterials (Basel). 2020 Dec 24;11(1):22. doi: 10.3390/nano11010022. Nanomaterials (Basel). 2020. PMID: 33374294 Free PMC article.
-
Long-term degradation of a poly-lactide co-glycolide/β-tricalcium phosphate biocomposite interference screw.Arthroscopy. 2011 May;27(5):637-43. doi: 10.1016/j.arthro.2010.11.056. Epub 2011 Mar 23. Arthroscopy. 2011. PMID: 21429700 Clinical Trial.
-
Evaluation of Hard and Soft Tissue Responses to Four Different Generation Bioresorbable Materials-Poly-l-Lactic Acid (PLLA), Poly-l-Lactic Acid/Polyglycolic Acid (PLLA/PGA), Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid (u-HA/PLLA) and Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid/Polyglycolic Acid (u-HA/PLLA/PGA) in Maxillofacial Surgery: An In-Vivo Animal Study.Materials (Basel). 2023 Nov 27;16(23):7379. doi: 10.3390/ma16237379. Materials (Basel). 2023. PMID: 38068124 Free PMC article.
-
Evaluation of Osseointegration and Bone Healing Using Pure-Phase β - TCP Ceramic Implant in Bone Critical Defects. A Systematic Review.Front Vet Sci. 2022 Jul 12;9:859920. doi: 10.3389/fvets.2022.859920. eCollection 2022. Front Vet Sci. 2022. PMID: 35909673 Free PMC article.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
Cited by
-
An evaluation of novel AMP2-coated electrospun composite scaffolds for intraoral bone regeneration: a proof-of-concept in vivo study.Front Bioeng Biotechnol. 2025 Apr 17;13:1443280. doi: 10.3389/fbioe.2025.1443280. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40313641 Free PMC article.
-
PRP and Cotton-Like β-TCP/PLGA Fibers Enhance Bone Repair in Osteoporotic Vertebral Defects via Macrophage Modulation in Rats.Acta Histochem Cytochem. 2025 Jun 24;58(3):123-132. doi: 10.1267/ahc.24-00066. Epub 2025 Jun 18. Acta Histochem Cytochem. 2025. PMID: 40630790 Free PMC article.
-
PRP and Cotton-Like β-TCP/PLGA Fibers Enhance Bone Repair in Osteoporotic Vertebral Defects via Macrophage Modulation in Rats.Acta Histochem Cytochem. 2025 Jun 24;58(3):123-132. doi: 10.1267/ahc.24-00066. Epub 2025 Jun 18. Acta Histochem Cytochem. 2025. PMID: 40630793 Free PMC article.
-
Enhancing Bone Repair with β-TCP-Based Composite Scaffolds: A Review of Design Strategies and Biological Mechanisms.Orthop Res Rev. 2025 Jul 14;17:313-340. doi: 10.2147/ORR.S525959. eCollection 2025. Orthop Res Rev. 2025. PMID: 40688750 Free PMC article. Review.
References
-
- Wu V., Helder M.N., Bravenboer N., Ten Bruggenkate C.M., Jin J., Klein-Nulend J., Schulten E.A.J.M. Bone Tissue Regeneration in the Oral and Maxillofacial Region: A Review on the Application of Stem Cells and New Strategies to Improve Vascularization. Stem Cells Int. 2019;30:6279721. doi: 10.1155/2019/6279721. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

