Functionalized Nanomaterials for Inhibiting ATP-Dependent Heat Shock Proteins in Cancer Photothermal/Photodynamic Therapy and Combination Therapy
- PMID: 38202567
- PMCID: PMC10780407
- DOI: 10.3390/nano14010112
Functionalized Nanomaterials for Inhibiting ATP-Dependent Heat Shock Proteins in Cancer Photothermal/Photodynamic Therapy and Combination Therapy
Abstract
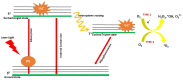
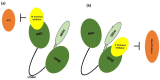
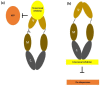
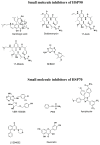
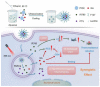
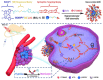
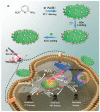
Phototherapies induced by photoactive nanomaterials have inspired and accentuated the importance of nanomedicine in cancer therapy in recent years. During these light-activated cancer therapies, a nanoagent can produce heat and cytotoxic reactive oxygen species by absorption of light energy for photothermal therapy (PTT) and photodynamic therapy (PDT). However, PTT is limited by the self-protective nature of cells, with upregulated production of heat shock proteins (HSP) under mild hyperthermia, which also influences PDT. To reduce HSP production in cancer cells and to enhance PTT/PDT, small HSP inhibitors that can competitively bind at the ATP-binding site of an HSP could be employed. Alternatively, reducing intracellular glucose concentration can also decrease ATP production from the metabolic pathways and downregulate HSP production from glucose deprivation. Other than reversing the thermal resistance of cancer cells for mild-temperature PTT, an HSP inhibitor can also be integrated into functionalized nanomaterials to alleviate tumor hypoxia and enhance the efficacy of PDT. Furthermore, the co-delivery of a small-molecule drug for direct HSP inhibition and a chemotherapeutic drug can integrate enhanced PTT/PDT with chemotherapy (CT). On the other hand, delivering a glucose-deprivation agent like glucose oxidase (GOx) can indirectly inhibit HSP and boost the efficacy of PTT/PDT while combining these therapies with cancer starvation therapy (ST). In this review, we intend to discuss different nanomaterial-based approaches that can inhibit HSP production via ATP regulation and their uses in PTT/PDT and cancer combination therapy such as CT and ST.
Keywords: cancer combination therapy; heat shock protein; nanomedicine; photodynamic therapy; photothermal therapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Key Modulation of ROS and HSP for Effective Therapy Against Hypoxic Tumor with Multifunctional Nanosystem.Int J Nanomedicine. 2023 Nov 18;18:6829-6846. doi: 10.2147/IJN.S432928. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38026539 Free PMC article.
-
Lonidamine liposomes to enhance photodynamic and photothermal therapy of hepatocellular carcinoma by inhibiting glycolysis.J Nanobiotechnology. 2023 Dec 15;21(1):482. doi: 10.1186/s12951-023-02260-z. J Nanobiotechnology. 2023. PMID: 38102658 Free PMC article.
-
Assembly of multifunction dyes and heat shock protein 90 inhibitor coupled to bovine serum albumin in nanoparticles for multimodal photodynamic/photothermal/chemo-therapy.J Colloid Interface Sci. 2021 May 15;590:290-300. doi: 10.1016/j.jcis.2021.01.052. Epub 2021 Jan 27. J Colloid Interface Sci. 2021. PMID: 33548612
-
Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine.ACS Nano. 2023 May 9;17(9):7979-8003. doi: 10.1021/acsnano.3c00891. Epub 2023 Apr 27. ACS Nano. 2023. PMID: 37129253 Free PMC article. Review.
-
Recent Progress of Copper-Based Nanomaterials in Tumor-Targeted Photothermal Therapy/Photodynamic Therapy.Pharmaceutics. 2023 Sep 7;15(9):2293. doi: 10.3390/pharmaceutics15092293. Pharmaceutics. 2023. PMID: 37765262 Free PMC article. Review.
Cited by
-
Determinants of Photodynamic Therapy Resistance in Cancer Cells.Int J Mol Sci. 2024 Nov 10;25(22):12069. doi: 10.3390/ijms252212069. Int J Mol Sci. 2024. PMID: 39596137 Free PMC article. Review.
-
HSF1 at the crossroads of chemoresistance: from current insights to future horizons in cell death mechanisms.Front Cell Dev Biol. 2025 Jan 9;12:1500880. doi: 10.3389/fcell.2024.1500880. eCollection 2024. Front Cell Dev Biol. 2025. PMID: 39850800 Free PMC article. Review.
-
Leveraging adenosine triphosphate for cancer theranostics.Theranostics. 2025 Mar 24;15(10):4708-4733. doi: 10.7150/thno.106291. eCollection 2025. Theranostics. 2025. PMID: 40225571 Free PMC article. Review.
-
Water-filtered infrared A radiation hyperthermia combined with immunotherapy for advanced gastrointestinal tumours.Cancer Med. 2024 Jul;13(14):e70024. doi: 10.1002/cam4.70024. Cancer Med. 2024. PMID: 39049187 Free PMC article.
-
The examination of in vitro photosensitizing efficacy of aloe-emodin loaded liposome following photodynamic therapy on melanoma cell lines.Sci Rep. 2025 Aug 6;15(1):28833. doi: 10.1038/s41598-025-14777-4. Sci Rep. 2025. PMID: 40770046 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

