Enantioselective Catalytic Aldol Reactions in the Presence of Knoevenagel Nucleophiles: A Chemoselective Switch Optimized in Deep Eutectic Solvents Using Mechanochemistry
- PMID: 38202587
- PMCID: PMC10779746
- DOI: 10.3390/molecules29010004
Enantioselective Catalytic Aldol Reactions in the Presence of Knoevenagel Nucleophiles: A Chemoselective Switch Optimized in Deep Eutectic Solvents Using Mechanochemistry
Abstract
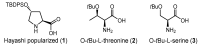
In the presence of different nucleophilic Knoevenagel competitors, cyclic and acyclic ketones have been shown to undergo highly chemoselective aldol reactions with aldehydes. In doing so, the substrate breadth for this emerging methodology has been significantly broadened. The method is also no longer beholden to proline-based catalyst templates, e.g., commercially available O-t-Bu-L-threonine is advantageous for acyclic ketones. The key insight was to exploit water-based mediums under conventional (in-water) and non-conventional (deep eutectic solvents) conditions. With few exceptions, high aldol-to-Knoevenagel chemoselectivity (>10:1) and good product profiles (yield, dr, and ee) were observed, but only in DESs (deep eutectic solvents) in conjunction with ball milling did short reaction times occur.
Keywords: Knoevenagel reaction; aldol reaction; deep eutectic solvents; green chemistry; in-water conditions; organic synthesis; organocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
An Extensive Study of Coumarin Synthesis via Knoevenagel Condensation in Choline Chloride Based Deep Eutectic Solvents.Curr Org Synth. 2020;17(2):98-108. doi: 10.2174/1570179417666200116155704. Curr Org Synth. 2020. PMID: 32418515
-
Towards the development of continuous, organocatalytic, and stereoselective reactions in deep eutectic solvents.Beilstein J Org Chem. 2016 Dec 5;12:2620-2626. doi: 10.3762/bjoc.12.258. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 28144332 Free PMC article.
-
Introducing deep eutectic solvents to polar organometallic chemistry: chemoselective addition of organolithium and Grignard reagents to ketones in air.Angew Chem Int Ed Engl. 2014 Jun 2;53(23):5969-73. doi: 10.1002/anie.201400889. Epub 2014 Apr 25. Angew Chem Int Ed Engl. 2014. PMID: 24771680
-
Deep eutectic solvents for biocatalytic transformations: focused lipase-catalyzed organic reactions.Appl Microbiol Biotechnol. 2020 Feb;104(4):1481-1496. doi: 10.1007/s00253-019-10342-y. Epub 2020 Jan 7. Appl Microbiol Biotechnol. 2020. PMID: 31907576 Review.
-
Can deep eutectic solvents be the best alternatives to ionic liquids and organic solvents: A perspective in enzyme catalytic reactions.Int J Biol Macromol. 2022 Sep 30;217:255-269. doi: 10.1016/j.ijbiomac.2022.07.044. Epub 2022 Jul 11. Int J Biol Macromol. 2022. PMID: 35835302 Review.
References
-
- Mahrwald R. Enantioselective Organocatalyzed Reactions. Springer; Dordrecht, The Netherlands: 2011.
-
- Berkessel A., Gröger H. Asymmetric Organocatalysis—From Biomimetic Concepts to Applications in Asymmetric Synthesis. Wiley-VCH; Weinheim, Germany: 2006.
-
- Dalko P. Enantioselective Organocatalysis. Wiley-VCH; Weinheim, Germany: 2007.
-
- Juaristi E. Recent Developments in Next Generation (S)-Proline-Derived Chiral Organocatalysts. Tetrahedron. 2021;88:132143. doi: 10.1016/j.tet.2021.132143. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources