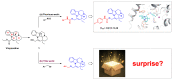
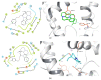
Synthesis and Activity Evaluation of Vinpocetine-Derived Indole Alkaloids
- PMID: 38202595
- PMCID: PMC10779641
- DOI: 10.3390/molecules29010014
Synthesis and Activity Evaluation of Vinpocetine-Derived Indole Alkaloids
Abstract
This study focuses on the synthesis of novel vinpocetine derivatives (2-25) and their biological evaluation. The chemical structures of the synthesized compounds were fully characterized using techniques such as 1H NMR, 13C NMR, and HRMS. The inhibitory activity of the synthesized compounds on PDE1A was evaluated, and the results revealed that compounds 3, 4, 5, 12, 14, 21, and 25 exhibited superior inhibitory activity compared to vinpocetine. Compound 4, with a para-methylphenyl substitution, showed a 5-fold improvement in inhibitory activity with an IC50 value of 3.53 ± 0.25 μM. Additionally, compound 25, with 3-chlorothiazole substitution, displayed an 8-fold increase in inhibitory activity compared to vinpocetine (IC50 = 2.08 ± 0.16 μM). Molecular docking studies were conducted to understand the binding models of compounds 4 and 25 within the active site of PDE1A. The molecular docking study revealed additional binding interactions, such as π-π stacking and hydrogen bonding, contributing to the enhanced inhibitory activity and stability of the ligand-protein complexes. Overall, the synthesized vinpocetine derivatives demonstrated promising inhibitory activity on PDE1A, and the molecular docking studies provided insights into their binding modes, supporting further development of these compounds as potential candidates for drug research and development.
Keywords: PDE1A; biological evaluation; molecular docking; structural modification; vinpocetine.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
Similar articles
-
Synthesis and biological evaluation of Vinpocetine derivatives.Bioorg Med Chem Lett. 2020 Jan 15;30(2):126472. doi: 10.1016/j.bmcl.2019.05.052. Epub 2019 May 28. Bioorg Med Chem Lett. 2020. PMID: 31859156
-
A novel PDE1A coupled to M2AChR at plasma membranes from bovine tracheal smooth muscle.J Recept Signal Transduct Res. 2016;36(3):278-87. doi: 10.3109/10799893.2015.1101136. Epub 2015 Oct 29. J Recept Signal Transduct Res. 2016. PMID: 26513204
-
Design, synthesis and docking study of novel tetracyclic oxindole derivatives as α-glucosidase inhibitors.Bioorg Med Chem Lett. 2015 Apr 1;25(7):1471-5. doi: 10.1016/j.bmcl.2015.02.031. Epub 2015 Feb 21. Bioorg Med Chem Lett. 2015. PMID: 25759031
-
An update on vinpocetine: New discoveries and clinical implications.Eur J Pharmacol. 2018 Jan 15;819:30-34. doi: 10.1016/j.ejphar.2017.11.041. Epub 2017 Nov 26. Eur J Pharmacol. 2018. PMID: 29183836 Free PMC article. Review.
-
[Neuroprotection strategies: effect of vinpocetine in vitro oxidative stress models].Acta Med Port. 2003 Nov-Dec;16(6):401-6. Epub 2003 Dec 1. Acta Med Port. 2003. PMID: 15631851 Review. Portuguese.
References
-
- Zhang J., You G., Zhang L. Sex differences in quality of life after ischemic stroke. Int. J. Stroke. 2018;13:146–147.
-
- Wang W., Jiang B., Sun H., Ru X., Sun D., Wang L., Wang L., Jiang Y., Li Y., Wang Y., et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480,687 Adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- ZK[2021] general 560/Guizhou Provincial Basic Research Program (Natural Science)
- ZK[2023] general 404/Guizhou Provincial Basic Research Program (Natural Science)
- K202222/the State Key Laboratory of Natural and Biomimetic Drugs
- Qian Jiao Ji [2023] 69/2023 Natural Science Research Project of Guizhou Provincial Department of Education
- QZYYZDXK(JS)-2021-03/Key Disciplines of Traditional Chinese Medicine and Ethnic Medicine in Guizhou Province during the 14th Five-Year Plan
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous