Synthetic Approaches to Piperazine-Containing Drugs Approved by FDA in the Period of 2011-2023
- PMID: 38202651
- PMCID: PMC10780301
- DOI: 10.3390/molecules29010068
Synthetic Approaches to Piperazine-Containing Drugs Approved by FDA in the Period of 2011-2023
Abstract
The piperazine moiety is often found in drugs or in bioactive molecules. This widespread presence is due to different possible roles depending on the position in the molecule and on the therapeutic class, but it also depends on the chemical reactivity of piperazine-based synthons, which facilitate its insertion into the molecule. In this paper, we take into consideration the piperazine-containing drugs approved by the Food and Drug Administration between January 2011 and June 2023, and the synthetic methodologies used to prepare the compounds in the discovery and process chemistry are reviewed.
Keywords: Buchwald–Hartwig amination; Finkelstein alkylation; amide bond formation; aromatic nucleophilic substitution; kinase inhibitors; receptor modulators; reductive amination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



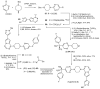
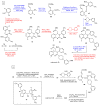


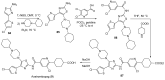
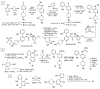

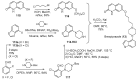

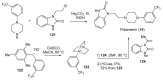
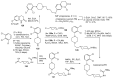
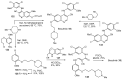
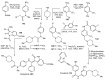

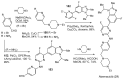
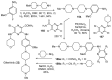

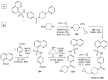



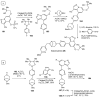




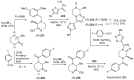

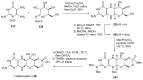
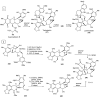
References
-
- Dinsmore C.J., Beshore D.C. Syntheses and transformations of piperazinone rings. A review. Org. Prep. Proced. Int. 2002;34:367–404. doi: 10.1080/00304940209458075. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

